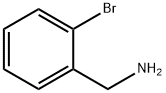
2-BROMOBENZYLAMINE synthesis
- Product Name:2-BROMOBENZYLAMINE
- CAS Number:3959-05-5
- Molecular formula:C7H8BrN
- Molecular Weight:186.05
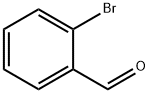
6630-33-7
402 suppliers
$9.00/10g

3959-05-5
159 suppliers
$5.00/1g
Yield:3959-05-5 97%
Reaction Conditions:
with [pentamethylcyclopentadienyl*Ir(N-phenyl-2-pyridinecarboxamidate)Cl];ammonium formate in methanol at 37; for 15 h;chemoselective reaction;
Steps:
General Procedure for Reductive Amination of Carbonyls – Isolated Product
General procedure: A carbonyl compound (0.5 mmol) and Ir1 (1 molpercent) were added to a 1.5-dram vial equippedwith a magnetic stir bar in methanol (2.5 mL). Solid HCOONH4 (5 mmol, 10 equiv.) was addedinto the vial and the solution was stirred at 37 °C for 15 h. The solvent was evaporated underreduced pressure and then aqueous HCl was added dropwise until a pH of 1-2 was obtained. Thesolution was washed with Et2O (3×5 mL), and the aqueous layer was collected. The aqueoussolution was adjusted to pH 10-12 using KOH. The product was extracted into DCM (3×5 mL)and the combined organic phase was dried over Na2SO4, filtered, and evaporated under reducedpressure to give the isolated product.
References:
Nguyen, Dat P.;Sladek, Rudolph N.;Do, Loi H. [Tetrahedron Letters,2020,vol. 61,# 32,art. no. 152196] Location in patent:supporting information
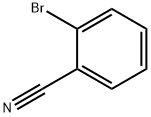
2042-37-7
350 suppliers
$7.00/10g

3959-05-5
159 suppliers
$5.00/1g

3433-80-5
307 suppliers
$10.00/1g

3959-05-5
159 suppliers
$5.00/1g

126799-87-9
4 suppliers
inquiry

3959-05-5
159 suppliers
$5.00/1g
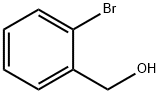
18982-54-2
277 suppliers
$5.00/5g

3959-05-5
159 suppliers
$5.00/1g