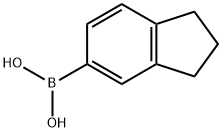
2,3-DIHYDRO-1H-INDEN-5-YLBORANEDIOL synthesis
- Product Name:2,3-DIHYDRO-1H-INDEN-5-YLBORANEDIOL
- CAS Number:196861-31-1
- Molecular formula:C9H11BO2
- Molecular Weight:161.99
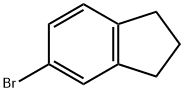
6134-54-9
52 suppliers
$125.00/250mg

196861-31-1
22 suppliers
inquiry
Yield:196861-31-1 57.2%
Reaction Conditions:
Stage #1: 5-bromoindanewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.25 h;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexane at -78 - -20; for 3 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexane at -20 - 20; for 2 h;
Steps:
5.1.4
10 g (0.0507 mol) of 5-bromo-2,3-dihydro-1H-indene thus obtained was stirred with 50 ml of dry THF with cooling to -78° C. To the resulting solution, 38.5 ml of a 1.6 M n-butyllithium in n-hexane was added dropwise. After the dropwise addition, the resulting solution was stirred at -78° C. for 15 minutes, and then 10.4 g (0.10 mol) of trimethyl borate was added dropwise. After the dropwise addition, the resulting solution was stirred for 3 hours while the temperature was allowed to elevate to -20° C. Then, 60 ml of 1 M hydrochloric acid was added dropwise, and the reaction solution was stirred for 2 hours while the temperature was allowed to elevate to room temperature. The reaction solution was extracted by adding 300 ml of ethyl acetate and 250 ml of saturated aqueous sodium chloride. The ethyl acetate layer was washed with saturated aqueous sodium chloride, and the ethyl acetate was evaporated under reduced pressure. The residual crystals were dispersed in 200 ml of n-hexane, collected by filtration and dried to obtain 4.7 g of the desired product (yield: 57.2%).
References:
US2012/209005,2012,A1 Location in patent:Page/Page column 32
496-11-7
240 suppliers
$5.00/5g

196861-31-1
22 suppliers
inquiry
6134-53-8
67 suppliers
$61.00/100mg

6134-54-9
52 suppliers
$125.00/250mg

196861-31-1
22 suppliers
inquiry
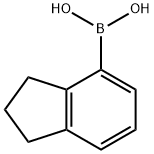
915411-11-9
3 suppliers
inquiry
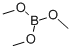
121-43-7
346 suppliers
$15.00/25ml

6134-53-8
67 suppliers
$61.00/100mg

6134-54-9
52 suppliers
$125.00/250mg

196861-31-1
22 suppliers
inquiry

915411-11-9
3 suppliers
inquiry