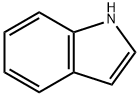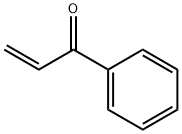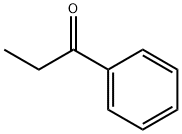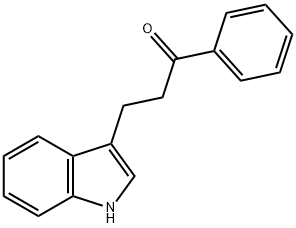
2-(3-indolyl)ethylphenyl ketone synthesis
- Product Name:2-(3-indolyl)ethylphenyl ketone
- CAS Number:13993-17-4
- Molecular formula:C17H15NO
- Molecular Weight:249.31
Yield:13993-17-4 75%
Reaction Conditions:
with montmorillonite K10 Clay in water at 80; for 12 h;Mechanism;Green chemistry;Solvent;Reagent/catalyst;
Steps:
3.2. General procedure for the alkylation of 1H-indole, 1H-benzimidazole, and 1H-benzotriazole
General procedure: A mixture of 1H -indole, 1H -benzimidazole, or 1H -benzotriazole (1.0 eq., 0.49 mmol), ketonic Mannich base(1.5 eq., 0.75 mmol), and montmorillonite K10 (0.15 eq., 75 mg) in 2 mL of water was heated to 80 °C. Thereaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to roomtemperature and extracted with EtOAc (3 ×10 mL) and then dried over MgSO 4 . Solvent was removed underreduced pressure and the crude product was purifi ed by column chromatography (EtOAc:hexane, 1:3; EtOAc). 3-(1H-Indol-3-yl)-1-phenylpropan-1-one (4a): White solid; mp 127-128 °C (lit. 29 126-127 °C);yield 75%; R f = 0.34 (EtOAc-hexane, 1:3); 1 H NMR (400 MHz, DMSO) δ 3.04 (t, J = 7.4 Hz, 2H), 3.42 (t, J = 7.4 Hz, 2H), 6.97 (t, J = 7.4 Hz, 1H), 7.06 (t, J = 7.0 Hz, 1H), 7.16 (bs, 1H), 7.32 (d, J = 8.0 Hz, 1H),7.49-7.56 (m, 3H), 7.63 (t, J = 6.8 Hz, 1H), 8.00 (d, J = 7.8 Hz, 2H), 10.78 (s, 1H); 13 C NMR (100 MHz,DMSO) δ = 19.9, 39.2, 111.8, 114.1, 118.7, 118.8, 121.4, 122.8, 127.5, 128.4, 129.2, 133.5, 136.7, 137.2, 200.2;HRMS (ESI): m/z calculated for C 17 H 16 NO [M+H] + : 250.1226; found: 250.1235.
References:
Seyi?Tdanlio?lu, P?nar;Hanashalshahaby, Essam Hamied Ahmed;ünalero?lu, Canan [Turkish Journal of Chemistry,2018,vol. 42,# 6,p. 1598 - 1610]
98-86-2
747 suppliers
$9.00/5g

13993-17-4
3 suppliers
inquiry