
2-(3-METHOXYPHENYL)QUINOLINE-4-CARBOXYLIC ACID synthesis
- Product Name:2-(3-METHOXYPHENYL)QUINOLINE-4-CARBOXYLIC ACID
- CAS Number:159782-19-1
- Molecular formula:C17H13NO3
- Molecular Weight:279.29
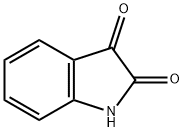
91-56-5
468 suppliers
$5.00/25g
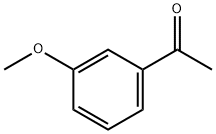
586-37-8
321 suppliers
$10.00/5g

159782-19-1
22 suppliers
$28.60/50mg
Yield:159782-19-1 43%
Reaction Conditions:
with potassium hydroxide in ethanol;lithium hydroxide monohydrate at 80;
Steps:
5.11 General procedure for preparation of 2-arylquinoline-4-carboxylic acid (11a-h)
General procedure: To a stirring mixtures of indoline-2,3-dione (3.1g, 0.02mol) in 33% potassium hydroxide solution (10mL), various substituted acetophenone (1.1equiv) in ethanol (30mL) was dropped slowly. The resulting reaction mixture was heated to 80°C for 24-72h, and monitored by TLC. The solvent was concentrated in vacuum and the residue was dissolved by water (100mL), which was extracted with diethyl ether (100mL×3), and the aqueous layer was acidified with glacial acetic acid to pH 5. The resulting precipitate was filtered, dried, and recrystallized with ethanol to yield a series of 2-arylquinoline-4-carboxylic acids 11a-h.
References:
Li, Sai;Huang, Qiang;Liu, Yajing;Zhang, Xiaolong;Liu, Shuang;He, Chao;Gong, Ping [European Journal of Medicinal Chemistry,2013,vol. 64,p. 62 - 73]

62-53-3
675 suppliers
$10.00/1g

159782-19-1
22 suppliers
$28.60/50mg