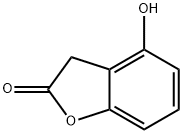
2(3H)-Benzofuranone, 4-hydroxy- synthesis
- Product Name:2(3H)-Benzofuranone, 4-hydroxy-
- CAS Number:2811-93-0
- Molecular formula:C8H6O3
- Molecular Weight:150.13
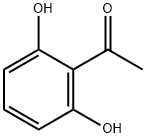
699-83-2
466 suppliers
$6.00/5g

2811-93-0
3 suppliers
inquiry
Yield:2811-93-0 46%
Reaction Conditions:
Stage #1: 2,6-dihydroxylacetophenonewith lithium hexamethyldisilazane in tetrahydrofuran at -78;Inert atmosphere;
Stage #2: with chloro-trimethyl-silane in tetrahydrofuran at -78; for 4 h;Inert atmosphere;
Steps:
B
Preparation of 4-hydroxybenzofuranone (Compound B); LiHMDS (1M solution in THF, 3.1 mL, 3.1 mmol, 3.6 eq.) was slowly added to a solution of 2',6'-dihydroxyacetophenone (131 mg, 0.86 mmol, 1 eq.) in anhydrous THF (4.5 mL) under argon atmosphere at -78° C. After 30 minutes, TMSCl (0.65 mL, 5.16 mmol, 6 eq.) was added and the resulting mixture was stirred for 4 hours. Then NBS (171 mg, 0.95 mmol, 1.1 eq.) was slowly added and the solution was stirred for 1 hour at -78° C. and for 10 minutes at rt. 1M NaOH (2 mL) was added and the resulting solution was stirred until complete disappearance of the starting material. The reaction was quenched by adding 1M HCl until pH 4. The aqueous layer was extracted with EtOAc and the collected organic extracts were washed with brine, dried on anhydrous Na2SO4 and evaporated under reduced pressure. The oily crude mixture was purified by silica gel column chromatography (eluent: EtOAc/petroleum ether 15:85). The title compound was obtained as a pale yellow solid. Yield: 46%. MS (m/z): 151.5 (MH+).
References:
US2009/311217,2009,A1 [] Location in patent:Page/Page column 51