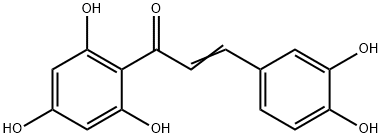
2',4',6',3,4-PENTAHYDROXYCHALCONE synthesis
- Product Name:2',4',6',3,4-PENTAHYDROXYCHALCONE
- CAS Number:73692-51-0
- Molecular formula:C15H12O6
- Molecular Weight:288.25
Yield:73692-51-0 55.3 kg
Reaction Conditions:
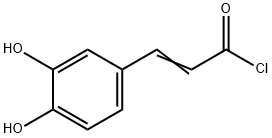
Stage #1: caffeic acid chloridewith aluminum (III) chloride in dichloromethane at 5 - 10; for 2 h;Large scale;
Stage #2: 3,5-dihydroxyphenol in dichloromethane at 10 - 20; for 4 h;Large scale;
Steps:
1 Synthesis of 1-caffeoyl-2,4,6 -trihydroxyphenol
In the 500L enamel reactor,200 L of dichloromethane and 40 g of caffeic acid chloride,Jacket cooling to about 5 , in part by adding anhydrous aluminum trichloride 13.5kg,And control the temperature at 5-10 , stirring reaction 2h, the formation of complexes.Continue to control the temperature within 10 , the batch of raw materials to join the phloroglucinol 26.0kg,And slowly rose to room temperature, and then stirring reaction 4h,TLC analysis of the raw material caffeic acid chloride complete reaction, as acylation reaction is completed.Cooling to 10 ° C or less, slowly adding 5% sodium hydroxide solution to pH 9 or so, hydrolysis 0.5h.And then 5% dilute hydrochloric acid to adjust the pH value to 6-7, standing stratification.The organic phase was separated and extracted again with 100 L of dichloromethane. The organic phases were combined,Dried, concentrated under reduced pressure and dried to give 55.3 kg of crude 1-cocoyl-2,4,6-trihydroxyphenol intermediate.
References:
CN103864743,2016,B Location in patent:Paragraph 0019; 0020
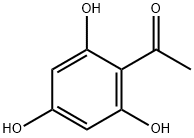
480-66-0
359 suppliers
$10.00/1g

73692-51-0
15 suppliers
inquiry
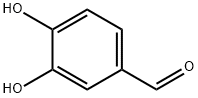
139-85-5
846 suppliers
$5.00/1g

73692-51-0
15 suppliers
inquiry
![2-Propen-1-one, 3-[3,4-bis(methoxymethoxy)phenyl]-1-[2,4,6-tris(methoxymethoxy)phenyl]-](/CAS/20210305/GIF/91299-69-3.gif)
91299-69-3
0 suppliers
inquiry

73692-51-0
15 suppliers
inquiry

![(Ethanone, 1-[2,4,6-tris(methoxymethoxy)phenyl]- )](/CAS/20180906/GIF/36804-11-2.gif)