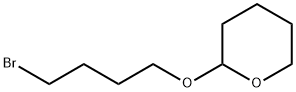
2-(4-Bromobutoxy)tetrahydro-2H-pyran synthesis
- Product Name:2-(4-Bromobutoxy)tetrahydro-2H-pyran
- CAS Number:31608-22-7
- Molecular formula:C9H17BrO2
- Molecular Weight:237.13

51326-51-3
15 suppliers
$205.00/2g

31608-22-7
68 suppliers
$75.00/1g
Yield:31608-22-7 80%
Reaction Conditions:
with 1H-imidazole;bromine;triphenylphosphine in toluene at 0 - 20; for 3 h;Inert atmosphere;Large scale;
Steps:
4 Example 3 Preparation of SLP-7b
In a 10-liter reactor, 640 g of SLP-6, 5 liters of toluene, 1.0 kg of triphenylphosphine, and 320 g of imidazole were added and the mixture was stirred until dissolution was complete. Under a nitrogen atmosphere, after the temperature was reduced to 0 degrees, 570 g of liquid was added in portions. The bromine was controlled so that the reaction temperature did not exceed 10 degrees; after the addition was complete, the reaction was warmed to room temperature for 3 hours until the end of the reaction.Add 3 liters of water and stir and let stand.The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to give the crude product; the crude product was purified by column chromatography on ethyl acetate/petroleum ether to give 697 g of SLP-7b (X=Br) as a pale yellow oil.Yield: 80%.H NMR (400MHz, CDCl3): δ 4.57 (m, 1H), 3.93-3.78 (m, 2H), 3.52-3.40 (m, 2H), 3.22 (t, 2H), 1.93-1.50 (m, 10H)ESI/MS+(m/z):237
References:
Shanghai Aikangrui Pharmaceutical Technology Co., Ltd.;Fan Linfeng;Zhu Yangwei;Qiu Aiyun;Lv Bojie;Xu Zhonghui;Zhang Changxuan CN106316967, 2017, A Location in patent:Paragraph 0063; 0079; 0080; 0081; 0082; 0083
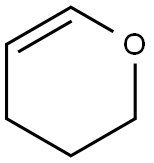
110-87-2
527 suppliers
$10.00/1g

33036-62-3
233 suppliers
$45.00/1g

31608-22-7
68 suppliers
$75.00/1g
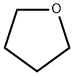
109-99-9
1061 suppliers
$9.00/5ml

110-87-2
527 suppliers
$10.00/1g

31608-22-7
68 suppliers
$75.00/1g

110-87-2
527 suppliers
$10.00/1g

31608-22-7
68 suppliers
$75.00/1g
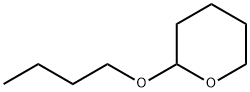
1927-68-0
18 suppliers
inquiry

31608-22-7
68 suppliers
$75.00/1g