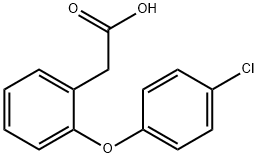
2-(4-Chlorophenoxy)phenylacetic acid synthesis
- Product Name:2-(4-Chlorophenoxy)phenylacetic acid
- CAS Number:25563-04-6
- Molecular formula:C14H11ClO3
- Molecular Weight:262.69
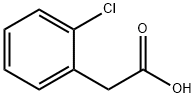
2444-36-2
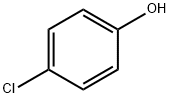
106-48-9

25563-04-6
1. 552.0 g (4.0 mol) of potassium carbonate was dissolved in 1500 mL of diethylene glycol dimethyl ether at 0-20 °C. 2. 283.1 g (2.2 mol) of p-chlorophenol and 5.74 g (0.04 mol) of copper (I) bromide were added sequentially to the above solution and stirred well. 3. the mixture was heated to 100 °C, 90.3 g (0.53 mol) of o-chlorophenylacetic acid was added, and the temperature was maintained and stirred for 1 hour. 4. 159.4 g (0.93 mol) of o-chlorophenylacetic acid was further added, the temperature was raised to 120-130 °C, stirring was continued for about 8 hours, and the progress of the reaction was monitored by high performance liquid chromatography. 5. Upon completion of the reaction, cooled to room temperature, 700mL of water and 650mL of 35wt% aqueous hydrochloric acid were added sequentially to adjust the pH of the aqueous layer to below 1. 6. After dilution by adding 500mL of water, the organic layer was extracted once with 400mL and 200mL of toluene, respectively, and the organic layer was combined. 7. The organic layer was sequentially washed three times with 500 mL of water and once with 500 mL of brine, and then dried with anhydrous magnesium sulfate. 8. The dried organic layer was partially concentrated to about 400 g of residue, 100 mL of heptane was added dropwise, and the internal temperature was maintained at about 70° C. until crystals precipitated. 9. Cooled to 20-25°C, filtered to collect the crystals and washed with 100mL of a solvent mixture of heptane/toluene (1:1). 10. Pressurized drying gave 245.6 g of 2-(2-(4-chlorophenoxy)phenyl)acetic acid white solid in 68.8% yield.
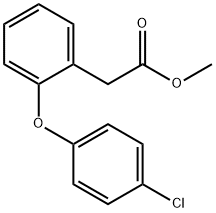
1037313-34-0
0 suppliers
inquiry

25563-04-6
59 suppliers
$41.00/100mg
Yield:25563-04-6 87%
Reaction Conditions:
Stage #1: 2-[(4-chloro-phenoxy)-phenyl]-acetic acid methyl esterwith potassium hydroxide in methanol at 60; for 3 h;
Stage #2: with hydrogenchloride in water; pH=1;
Steps:
9.A
Example 9. Preparation of asenapine via the lactam trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1 H-dibenz [2,3:6,7] oxepino [4,5-c] pyrrol-1-one (VII). A: [2-(4-Chloro-phenoxy)-phenyl-acetic acid (IX) Methyl 2-bromophenylacetate (47.0 grams 216.3 mmol) and 4-chlorophenol (27.8 grams, 215.8 mmol) were dissolved in dioxane (790 ml) while warming to 50 °C. To the resulting solution were added, under stirring in an inert nitrogen atmosphere, cesium carbonate (141 grams, 432.2 mmol) and copper(I) chloride (18.56 g, 86.2 mmol). Finally, N,N-dimethylglycine (4.46 grams, 43.2 mmol) was added to the green suspension. The reaction mixture was heated at 110 °C (reflux temperature) for 4 days while stirring. The reaction mixture was filtered over dicalite, which was washed with dioxane (40 ml). The dioxane was removed in vacuo to leave a brownish oil. Ethyl acetate (100 ml) was added to the oil and the pH of the resulting mixture was adjusted to 1 by addition of 1 M HCl (300 ml). The organic phase was washed with saturated brine (300 ml), dried on magnesium sulphate and then concentrated under vacuum to yield 57.2 grams (206 mmol; 95 %) of 2-(4-chloro-phenoxy)-phenyl]-acetic acid methyl ester as a brown oil. The oil was dissolved in methanol (250 ml), whereupon KOH (16.2 grams; 1.4 eq) was added. The resulting solution was heated for 3 h at 60 °C. The reaction mixture was cooled and the methanol (200 ml) was removed under vacuum. To the residue water (100 ml) was added and the pH was adjusted to 1 by addition of 1 M HCl (25 ml). The resulting crystals were filtered off and dried in vacuum at 40 °C to yield 47.3 grams (180 mmol; 87 %) of [2-(4-chloro-phenoxy)-phenyl]-acetic acid (IX).
References:
EP1710241,2006,A1 Location in patent:Page/Page column 10
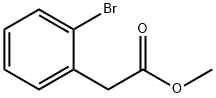
57486-69-8
141 suppliers
$6.00/1g

106-48-9
498 suppliers
$10.00/5G

25563-04-6
59 suppliers
$41.00/100mg

2444-36-2
461 suppliers
$5.00/25g

106-48-9
498 suppliers
$10.00/5G

25563-04-6
59 suppliers
$41.00/100mg
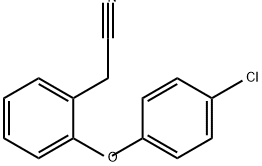
25958-86-5
0 suppliers
inquiry

25563-04-6
59 suppliers
$41.00/100mg
![Ethanethione, 2-[2-(4-chlorophenoxy)phenyl]-1-(4-morpholinyl)-](/CAS/20210305/GIF/345308-89-6.gif)
345308-89-6
0 suppliers
inquiry

25563-04-6
59 suppliers
$41.00/100mg