2-(4-chlorophenyl)-2-methylpropanenitrile synthesis
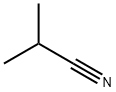
- Product Name:2-(4-chlorophenyl)-2-methylpropanenitrile
- CAS Number:30568-32-2
- Molecular formula:C10H10ClN
- Molecular Weight:179.65
Yield:30568-32-2 90.7%
Reaction Conditions:
with lithium dipropan-2-ylazanide in tetrahydrofuran;n-heptane;
Steps:
1 Preparation of p-Chloro-α-methylhydratroponitrile STR12
EXAMPLE 1 Preparation of p-Chloro-α-methylhydratroponitrile STR12 A solution of (p-chlorophenyl)acetonitrile (30.32 g, 0.20 mol) in tetrahydrofuran is treated dropwise with lithium diisopropylamide (0.44 mol, 220 mL of a 2M solution in heptane/tetrahydrofuran/benzene) at -25° C. to -30° C., over 60 minutes under nitrogen, stirred at -15° C. for one hour, treated dropwise with a solution of iodomethane (62.45 g, 0.44 mol) in tetrahydrofuran at -15° C., stirred at -15° C. for one hour, and diluted with water. The aqueous solution is extracted with ether. The organic extract is washed sequentially with water, 2N hydrochloric acid and water, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain an oily residue. The residue is distilled to give the title product as a colorless oil (32.6 g, bp 89-91° C./1 mm Hg, 90.7% yield).
References:
US5998673,1999,A

74-88-4
340 suppliers
$15.00/10g

30568-32-2
20 suppliers
inquiry

149-73-5
519 suppliers
$12.00/5g
140-53-4
477 suppliers
$13.00/25g

30568-32-2
20 suppliers
inquiry
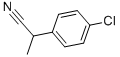
2184-88-5
21 suppliers
inquiry