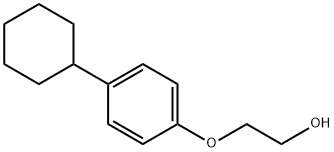
2-(4-Cyclohexylphenoxy)ethanol synthesis
- Product Name:2-(4-Cyclohexylphenoxy)ethanol
- CAS Number:1020-00-4
- Molecular formula:C14H20O2
- Molecular Weight:220.31
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1020-00-4
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;palladium dihydroxide in ethanol at 20; for 25 h;
Steps:
35.b Production example 35b)1-(2-Hydroxyethoxy)-4-cyclohexylbenzene
3.2 g of 1-[2-(Benzyloxy)ethoxy)-4-cyclohexylbenzene was dissolved in 100 ml of ethanol, and 300 mg of 20% palladium hydroxide was added, and the mixture was stirred at room temperature under hydrogen atmosphere for 25 hours. The catalyst was filtered off and washed with ethyl acetate. The filtrate was evaporated, and the residue was subjected to azeotropy with toluene (×2), to give 2.34 g of the title compound as a colorless solid.1H-NMR (CDCl3) δ : 1.17-1.44 (m, 5H) 1.70-1.90 (m, 5H) 2.40-2.50 (m, 1H) 3.62 (t, J=6.4Hz, 2H) 4.27 (t, 3=6.4Hz, 2H) 6.82-6.87 (m, 2H) 7.11-7.15 (m, 2H)
References:
Eisai Co., Ltd. EP1380562, 2004, A1 Location in patent:Page 62
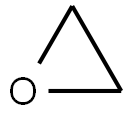
75-21-8
219 suppliers
$33.29/crm44609
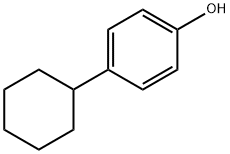
1131-60-8
180 suppliers
$9.00/5g

1020-00-4
1 suppliers
inquiry
