
2,4-Diamino-5-Iodo-6-Methylpyrimidine synthesis
- Product Name:2,4-Diamino-5-Iodo-6-Methylpyrimidine
- CAS Number:189810-94-4
- Molecular formula:C5H7IN4
- Molecular Weight:250.04

1791-73-7
71 suppliers
$45.00/10mg

189810-94-4
19 suppliers
$362.00/500mg
Yield:189810-94-4 89%
Reaction Conditions:
with Iodine monochloride;acetic acid at 20; for 3 h;
Steps:
8 5-iodo-6-methyl-pyrimidine-2,4-diamine (6):
To a solution of AB-323/25048181 (1.50g, 12.06mmol) in 15mL AcOH a solution of ICI (2.54g, 15.64mmol) in 15mL AcOH was added. The reaction mixture was stirred at room temperature for 3h after which TLC (EtOAc) indicated complete consumption of the starting material. The reaction was basified with a 4N NaOH solution and extracted twice with EtOAc. The combined organic layers are washed with brine, dried over MgSO4 and evaporated to give 6 (2.70g, 10.80mmol, 89%) as a brown solid. 1H NMR (400 MHz, CDCl3) δ ppm 5.14 (br. s., 2H), 4.70 (br. s., 2H), 2.44 (s, 3H).
References:
EP3811945,2021,A1 Location in patent:Paragraph 0064-0065; 0069
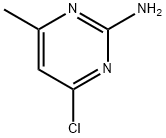
5600-21-5
318 suppliers
$18.00/25g

189810-94-4
19 suppliers
$362.00/500mg