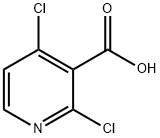
2,4-Dichloropyridine-3-carboxylic acid synthesis
- Product Name:2,4-Dichloropyridine-3-carboxylic acid
- CAS Number:262423-77-8
- Molecular formula:C6H3Cl2NO2
- Molecular Weight:192
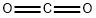
124-38-9
122 suppliers
$175.00/23402

262423-77-8
200 suppliers
$9.00/1g
Yield:262423-77-8 82%
Reaction Conditions:
Stage #1:2,4-dichloropyridine with n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2:carbon dioxide in tetrahydrofuran;hexane at -78 - 20; for 0.166667 h;
Stage #3: with hydrogenchloride;water; pH=4
Steps:
Preparation of 2,4-Dichloronicotinic acid To a stirring solution of diisopropyl ethyl amine (11.1 ml, 81.08 mmol) in THF (50 ml) was added dropwise a solution of BuLi (1.46 M, 43.3 ml, 73.65 mmol) in hexane below -65° C. and the mixture was stirred for 40 minutes. To this solution was added dropwise 2,4-dichloropyridine (10 g, 67.57 mmol) in THF (15 mL) at -78° C. and stirred for 30 minutes. Carbon dioxide generated from freshly crushed dry ice was passed through CaCl2 guard tube and then charged into the reaction mixture for 10 minutes and the reaction mixture was slowly allowed to come to room temperature. The solvent was evaporated under reduced pressure and dissolved in a minimum volume of water. The aqueous layer was washed with water and acidified to pH 4 with conc. HCl. It was then extracted with ethyl acetate, the organic layer was washed with brine and dried over sodium sulfate. The organic solvent was removed under reduced pressure to provide 2,4-dichloronicotinic acid (10.6 g, 82%) as off white solid. 1H NMR (400 MHz, DMSO-d6): δ 14.88-14.54 (br s, 1H), 8.46 (d, J=5.3 Hz, 1H), 7.74 (d, J=5.5 Hz, 1H). FIA MS [M+H]: 191.8
References:
Pfizer Inc US2008/85887, 2008, A1 Location in patent:Page/Page column 17
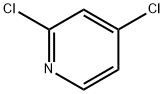
26452-80-2
404 suppliers
$7.00/5g
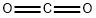
124-38-9
122 suppliers
$175.00/23402

262423-77-8
200 suppliers
$9.00/1g

26452-80-2
404 suppliers
$7.00/5g

262423-77-8
200 suppliers
$9.00/1g
109-09-1
11 suppliers
$10.60/1gm:

262423-77-8
200 suppliers
$9.00/1g
153034-86-7
362 suppliers
$10.00/1g

262423-77-8
200 suppliers
$9.00/1g