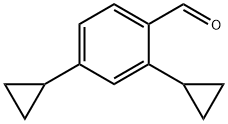
2,4-dicyclopropylbenzaldehyde synthesis
- Product Name:2,4-dicyclopropylbenzaldehyde
- CAS Number:1245645-99-1
- Molecular formula:C13H14O
- Molecular Weight:186.25
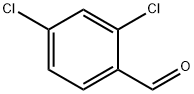
874-42-0
420 suppliers
$6.00/25g
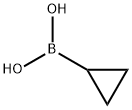
411235-57-9
436 suppliers
$6.00/1g

1245645-99-1
9 suppliers
inquiry
Yield:1245645-99-1 68%
Reaction Conditions:
with potassium phosphate tribasic heptahydrate;C45H53ClFeNO2PPd in water;toluene at 100; for 5.5 h;Inert atmosphere;Suzuki coupling;
Steps:
4.2. General experimental procedure
General procedure: Potassium phosphate (0.75 mmol) and IIe (1 mol %) was added to the solution of aryl halides (0.25 mmol) and cyclopropylboronic acid (0.5 mmol) in toluene (2.0 mL) and water (100 μL). The mixture was heated to 100 °C for a proper time under nitrogen atmosphere and cooled to room temperature. Water (10 mL) was added and the mixture was extracted with EtOAc (3×15 mL), evaporated and purified by chromatography on silica gel.
References:
Zhang, Min;Cui, Xiuling;Chen, Xiaopei;Wang, Lianhui;Li, Jingya;Wu, Yusheng;Hou, Lifen;Wu, Yangjie [Tetrahedron,2012,vol. 68,# 3,p. 900 - 905] Location in patent:experimental part