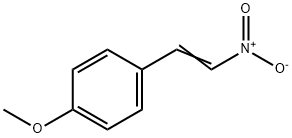
2-(4-METHOXYPHENYL)-3-NITRO-2H-CHROMENE synthesis
- Product Name:2-(4-METHOXYPHENYL)-3-NITRO-2H-CHROMENE
- CAS Number:57544-02-2
- Molecular formula:C16H13NO4
- Molecular Weight:283.28
Yield:57544-02-2 76%
Reaction Conditions:
with pyrrolidine;benzoic acid in ethanol; for 12 h;Reflux;Inert atmosphere;
Steps:
Typical procedure for the preparation of chromenes 3
General procedure: To a salicylaldehyde 1a (1.1 mmol), β-nitrostyrene 2a (1.0 mmol), pyrrolidine (0.30 mmol) andbenzoic acid (0.30 mmol) in 2.0 mL of anhydrous EtOH were heated at reflux for 12 h under aninert atmosphere. The reaction was monitored by thin-layer chromatography (eluent: nhexane/EtOAc 50:1, V/V). After the reaction finished, the solvent was removed under reducedpressure and the residue was purified by flash column chromatography (eluent: n-hexane/EtOAc50:1, V/V) to provide pure chromene 3a as yellow crystals. Chromenes 3a-i are knowncompounds.
References:
Wang, Ping-An;Zhang, Dong-Xu;Liu, Xue-Ying [ARKIVOC,2014,vol. 2014,# 5,p. 408 - 419]
123-11-5
841 suppliers
$10.00/10g

57544-02-2
8 suppliers
inquiry