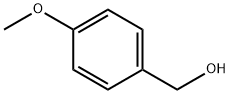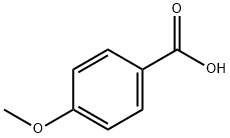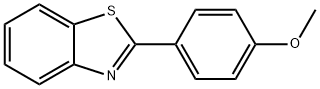
2-(4-METHOXYPHENYL)BENZOTHIAZOLE synthesis
- Product Name:2-(4-METHOXYPHENYL)BENZOTHIAZOLE
- CAS Number:6265-92-5
- Molecular formula:C14H11NOS
- Molecular Weight:241.31
Yield: 88%
Reaction Conditions:
with 3-nitropyridine;sodium t-butanolate in dimethyl sulfoxide at 110; for 16 h;
Steps:
General experimental procedure for the synthesis of products benzoxazoles (3a-v), naphthoxazoles (5a-f), benzothiazoles (8a-f) and benzimidazoles (9a-c) using 3-nitropyridine as organocatalyst.
General procedure: A 25 mL RB was charged with a mixture of 2-aminophenols 1a-h (1.0 mmol) or 1-amino-2-naphthol 4a (1.0 mmol) or 2-aminothiophenol 6a (1.0 mmol) or o-phenylenediamine 7a (1.0 mmol) and benzyl alcohols 2a-e, g-j or cinnamyl alcohol 2f (1.0 mmol) along with 3-nitropyridine (0.1 mmol, 12.4 mg), NaOtBu (1.0 mmol, 96 mg) and DMSO (2 mL). The RB was loosely fitted with septum and then heated at 110 °C for 16 h. After completion of the reaction, the mixture was diluted with hot ethyl acetate (20 mL) and water (40 mL) and extracted with ethyl acetate (3 10 mL). The combined organic layer was washed with brine (2 10 mL) and dried over anhydrous Na2SO4. Solvent was removed under reduced pressure and the remaining residue was purified by flash chromatography over silica gel using hexane / ethyl acetate = 9:1 (v/v) as an eluent to obtain the desired products benzoxazoles 3a-v, naphthoxazoles 5a-f, benzothiazoles 8a-f and benzimidazoles 9a-c in high yields.
References:
Kaldhi, Dhananjaya;Vodnala, Nagaraju;Gujjarappa, Raghuram;Nayak, Subhashree;Ravichandiran;Gupta, Sreya;Hazra, Chinmoy K.;Malakar, Chandi C. [Tetrahedron Letters,2019,vol. 60,# 3,p. 223 - 229] Location in patent:supporting information
2393-23-9
461 suppliers
$20.00/25g
137-07-5
290 suppliers
$10.00/5g

6265-92-5
64 suppliers
$15.00/1g

26060-23-1
2 suppliers
inquiry

6265-92-5
64 suppliers
$15.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6265-92-5
64 suppliers
$15.00/1g