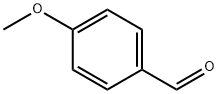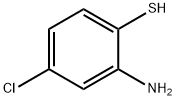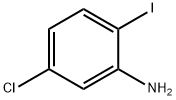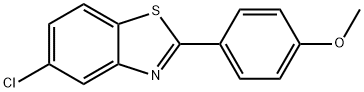
5-CHLORO-2-(4-METHOXYPHENYL)BENZO[D]THIAZOLE synthesis
- Product Name:5-CHLORO-2-(4-METHOXYPHENYL)BENZO[D]THIAZOLE
- CAS Number:92161-46-1
- Molecular formula:C14H10ClNOS
- Molecular Weight:275.75
Yield:92161-46-1 90%
Reaction Conditions:
with fluorescein in methanol at 20;Irradiation;
Steps:
4.2. General procedure for synthesis of products 3a-3t
General procedure: 2-Aminothiophenol derivatives 1 (1 mmol), aromatic aldehyde 2 (1mmol) and fluorescein (10 mol%) were dissolved in 20 mL methanol andplaced in a flat quartz glass jar. The open-air reaction container wasplaced under a 30 W blue LED lamp. After completion of the reaction(monitored by TLC analysis), the solvent was removed under reducedpressure. Then, water (20 mL) was added and the mixture was extractedwith ethyl acetate (3 × 20 mL). The combined organic layer was driedover anhydrous Na2SO4 and the solvent was removed in vacuo. Thecrude product was purified via silica gel column chromatography (petroleumether/ethyl acetate rations of 4:1-6:1) to generate the corresponding product 3.2-Phenylbenzothiazole (3a). 92% yield as a white solid
References:
Chen, Haodong;Lei, Mayan;Liu, Chunyan;Sun, Wuji;Wang, Kaixuan;Wang, Xueyao;Zhong, Qidi [Molecular catalysis,2021,vol. 510]