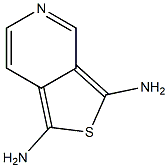
2,5-Benzothiazolediamine(9CI) synthesis
- Product Name:2,5-Benzothiazolediamine(9CI)
- CAS Number:50480-29-0
- Molecular formula:C7H7N3S
- Molecular Weight:165.22
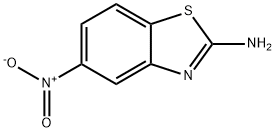
73458-39-6
81 suppliers
$28.60/5MG

50480-29-0
30 suppliers
$45.00/50mg
Yield:50480-29-0 66%
Reaction Conditions:
with ethanol;tin(ll) chloride at 80; for 4 h;
Steps:
16 Example 16:; 5-amino-benzothiazol-2-ylamine
To a solution of ethanol (36 mL) was added [5-NITRO-BENZOTHIAZOL-2-YLAMINE] (Example 15,78 mg, [O 35 MMOL)] and tin dichloride dihydrate (449 [MG,] 2 [MMOL).] The resulting mixture was heated at [80C] for 4 hours. The solution was cooled to room temperature and the solvent was removed. The residue was dissolved in ethyl acetate and poured onto 50 mL of 1.5 N [NAOH] solution and extracted using ethyl acetate (3 x 30 mL). The combined organic layers were dried over [MGS04,] filtered and concentrated to give a solid (43 mg, [66%). 1H] NMR (DMSO d) 8 : 7.23-7. 19 (m, 3H), 6.59 (d, [1H,] J = 1.96 Hz), 6.32 (dd, [1 H,] J = 2.18, 8.28 Hz), 4.88 [(BR S,] 2H); MS [(ESI)] 166 [(M+1,] 100%).
References:
WO2004/14885,2004,A1 Location in patent:Page 35
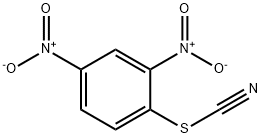
1594-56-5
87 suppliers
$49.00/1g

50480-29-0
30 suppliers
$45.00/50mg
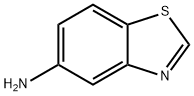
1123-93-9
153 suppliers
$6.00/250mg

50480-29-0
30 suppliers
$45.00/50mg
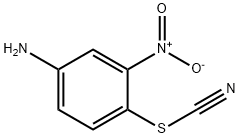
23153-15-3
0 suppliers
inquiry

50480-29-0
30 suppliers
$45.00/50mg
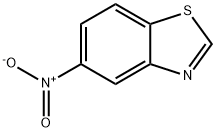
2942-07-6
103 suppliers
$9.00/100mg

50480-29-0
30 suppliers
$45.00/50mg