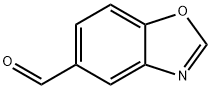
5-Benzoxazolecarboxaldehyde (9CI) synthesis
- Product Name:5-Benzoxazolecarboxaldehyde (9CI)
- CAS Number:638192-65-1
- Molecular formula:C8H5NO2
- Molecular Weight:147.13

1622428-38-9
0 suppliers
inquiry

638192-65-1
74 suppliers
$53.00/50mg
Yield:638192-65-1 33.9%
Reaction Conditions:
Stage #1: 5-vinylbenzo[d]oxazolewith ozone in dichloromethane at -78; for 3652.5 h;Inert atmosphere;
Stage #2: with triethylamine in dichloromethane at 25; for 1 h;
Steps:
3 Preparation of benzo[d]oxazole-5-carbaldehyde (LXI)
To a solution of 5-vinylbenzo[d]oxazole (LX) (6.1 g, 42.1 mmol, 1.0 eq) in DCM (100 mL) was bubbled ozone at -78° C. until the solution turn to blue. The solution was then purged with O2 followed by N2 for 5 minutes. TEA (12.8 g, 126.3 mmol, 3 eq) was added and the mixture was stirred at 25° C. for 1 h. The solution was poured into water (200 mL) and extracted with EtOAc (3×300 mL). The combined organic phase was dried, filtered and concentrated in vacuo. The residue was purified by chromatography on silica gel eluted (PE:EtOAc=10:1→1:1) to give benzo[d]oxazole-5-carbaldehyde (LXI) (2.1 g, 14.3 mmol, 33.9%) as a colorless oil. 1H NMR (CDCl3, 400 MHz) δ ppm 7.75 (d, J=8.4 Hz, 1H), 8.02 (dd, J=8.4 Hz, J=1.2 Hz, 1H), 8.22 (s, 1H), 8.33 (s, 1H), 10.13 (s, 1H); ESIMS found C8H5NO2 m/z 148.0 (M+H).
References:
US2014/243349,2014,A1 Location in patent:Paragraph 1741; 1744

40925-68-6
257 suppliers
$6.00/1g

638192-65-1
74 suppliers
$53.00/50mg
132244-31-6
149 suppliers
$14.00/500mg

638192-65-1
74 suppliers
$53.00/50mg

15112-41-1
83 suppliers
$22.00/100mg

638192-65-1
74 suppliers
$53.00/50mg