
2,5-diaminobenzene sulfonamide synthesis
- Product Name:2,5-diaminobenzene sulfonamide
- CAS Number:20896-44-0
- Molecular formula:C6H9N3O2S
- Molecular Weight:187.22
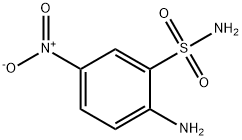
54734-85-9
18 suppliers
inquiry

20896-44-0
18 suppliers
inquiry
Yield:20896-44-0 98%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in tetrahydrofuran;methanol at 23; under 2585.81 Torr;autoclave;Inert atmosphere;Industry scale;
Steps:
1.c
2-Amino-5-nitro-benzenesulfonamide (5.00 kg, 23.0 mol), methanol(65 L), tetrahydrofuran (65 L), and 10% palladium on carbon (250 g) were charged to an autoclave. The mixture was cycled with nitrogen and hydrogen purges (3 x), and the mixture was then stirred under hydrogen (50 psi) at 23 0C overnight. The catalyst was removed by filtration and the filtrate was then concentrated in vacuo to give a brown solid. The solid was further dried in vacuo at 45 0C to afford the desired product, 2,5-diamino-benzenesulfonamide (4.21 kg, 22.4 mol, 98%), as a solid. 1H NMR (400 MHz, OMSO-d6) δ: 4.54 (2H, bs), 4.98 (2H, bs), 6.55 - 6.60 (2H, m), 6.87 (IH, d, J= 2.2 Hz), 6.99 (2H, bs). LC-MS (ESI) calcd for C6H9N3O2S 187.04, found 188.3 [M+H+].
References:
WO2010/42834,2010,A1 Location in patent:Page/Page column 36-37
![Benzenesulfonamide, 5-nitro-2-[(phenylmethyl)amino]-](/CAS/20210305/GIF/1000313-17-6.gif)
1000313-17-6
0 suppliers
inquiry

20896-44-0
18 suppliers
inquiry
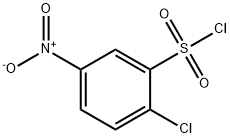
4533-95-3
124 suppliers
$9.00/100mg

20896-44-0
18 suppliers
inquiry
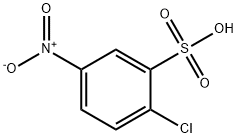
96-73-1
110 suppliers
inquiry

20896-44-0
18 suppliers
inquiry
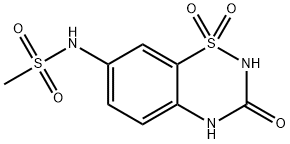
![Benzenesulfonamide, 2-amino-5-[(methylsulfonyl)amino]-](/CAS/20210305/GIF/691361-76-9.gif)