
2,5-DIMETHOXY-4-METHYLBENZALDEHYDE synthesis
- Product Name:2,5-DIMETHOXY-4-METHYLBENZALDEHYDE
- CAS Number:4925-88-6
- Molecular formula:C10H12O3
- Molecular Weight:180.2
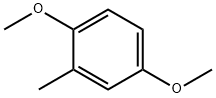
24599-58-4
138 suppliers
$15.00/1g
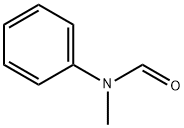
93-61-8
294 suppliers
$10.00/5 g

4925-88-6
90 suppliers
$42.39/1g
Yield:4925-88-6 67%
Reaction Conditions:
Stage #1:N-methyl-N-phenylformamide with trichlorophosphate at 20; for 0.666667 h;
Stage #2:2,5-dimethoxy toluene at 50; for 6 h;
Stage #3: with sodium acetate;sodium hydrogensulfitemore than 3 stages;
Steps:
A.a Preparation of the Syntetic Intermediates; A. Preparation of the Acids 1 (Variants); 2,5-Dimethoxy-4-methylbenzoic Acid (Compound A.1); a) 2,5-Dimethoxy-4-methylbenzaldehyde
After stirring a mixture of 8.5 mi of N-methyifor maniiide (0.068 moi) and 6.3 mi of phosphorus oxytrichio ride (0.068 moi) at room temperature for 40 minutes, 17.8 g of 2,5-dimethoxytoiuene (0.117 moi) are introduced. The reaction mixture is heated for 6 hours at 500 C. and then, after returning to a temperature of 20 C., it is hydroiysed with 100 mi of aqueous 10% sodium acetate soiution, extracted twice with diethyi ether and concentrated. The residue is taken up in aqueous sodium hydrogen suiphite soiution and extracted twice with diethyi ether. The aqueous phase is basied (pH=12) in order to give white crystais; m.p.=83 C.; yieid=67%.
References:
Bignon, Eric;Bras, Jean Pierre;De Cointet, Paul;Despeyroux, Pierre;Frehel, Daniel;Gully, Danielle US2004/43995, 2004, A1 Location in patent:Page 12
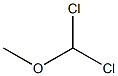
4885-02-3
190 suppliers
$10.00/1g

24599-58-4
138 suppliers
$15.00/1g

4925-88-6
90 suppliers
$42.39/1g
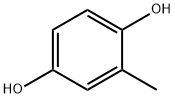
95-71-6
409 suppliers
$9.00/25g

4925-88-6
90 suppliers
$42.39/1g

24599-58-4
138 suppliers
$15.00/1g
557-21-1
105 suppliers
$12.00/5g

4925-88-6
90 suppliers
$42.39/1g

