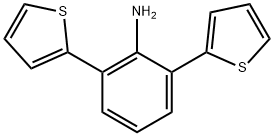
2,6-di(thiophen-2-yl)aniline synthesis
- Product Name:2,6-di(thiophen-2-yl)aniline
- CAS Number:1415512-67-2
- Molecular formula:C14H11NS2
- Molecular Weight:257.37
Yield:1415512-67-2 87.3%
Reaction Conditions:
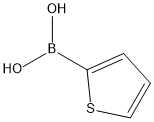
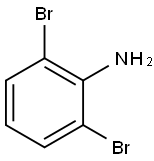
Stage #1: thiophene boronic acid;2,6-dibromoanilinewith bis-triphenylphosphine-palladium(II) chloride in 1,4-dioxane at 20; for 0.5 h;Inert atmosphere;Suzuki Coupling;
Stage #2: with water;potassium carbonate in 1,4-dioxane at 90; for 72 h;Inert atmosphere;
Steps:
Synthesis of 2,6-di (thiophen-2-yl) aniline (DOTA, Figure 3)
A mixture made of 0.502 g (2 mmol) 2,6-dibromoaniline, 0.768 g (6 mmol)thiophene-2-ylboronic acid, 0.210 g (0.3 mmol, 15%) bis (triphenylphosphine)-palladium(II)chloride and 20 mL of dioxane was stirred under argon for 30min at room temperature. 8 mL of aqueous 1 molL-1 potassium carbonate wasadded and the mixture was heated for 72 h at 90 °C . After cooling and addingwater, the mixture is extracted with ethyl acetate, dried with anhydrous sodiumsulfate and vacuum filtered. After separating the compound by elution of thesample with a 95 % n-hexane and 5 % ethyl acetate mixture, 0.449 g (yield:87.3 %) of light yellow oil was afforded. After recrystallization a pale yellowsolid was obtained. IR 3483.44 (N-H), 1449.93 cm-1 (C-S).1H-NMR (400 MHz, CDCl3), δ 7.48 (m, 4H, H4-H6), 7.42 (dd, 2H, H8),7.28 (dd, 2H, H7), 7.00 (t, 1H, H5), 4.55 (s, 2H, H1) ppm, 13C-NMR (400MHz, CDCl3), δ 142.84(C2), 141.60(C9), 131.66(C6), 128.30(C4), 126.92(C7), 126.21(C8), 121.03(C5), 118.58(C3) ppm.
References:
Ortega;Armijo;Jessop;Del Valle;Diaz [Journal of the Chilean Chemical Society,2013,vol. 58,# 4,p. 1959 - 1962]