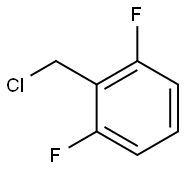
2,6-Difluorobenzyl chloride synthesis
- Product Name:2,6-Difluorobenzyl chloride
- CAS Number:697-73-4
- Molecular formula:C7H5ClF2
- Molecular Weight:162.56
2,6-Difluorobenzyl chloride is used as rufinamide intermediate. It is an important raw material and intermediate used in organic synthesis. It was prepared from 2,6-Difluorobenzyl alcohol by stirring in hydrogenchloride for 1h at 110℃.
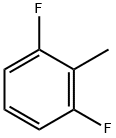
443-84-5
220 suppliers
$11.00/1g

697-73-4
290 suppliers
$6.00/1g
Yield:697-73-4 43%
Reaction Conditions:
with methanol;tetrachloromethane;iron(II) chloride tetrahydrate;formamide at 180; for 6 h;Inert atmosphere;Sealed tube;Autoclave;chemoselective reaction;Reagent/catalyst;
Steps:
4.2. Preparation of mono- and difluorobenzyl chlorides. 4.2.1. General procedures. Method A.
General procedure: The reactions were carried out in a glass tube (V=10 mL) placed into a stainless steel autoclave (V=17 mL) at a controlled heating. The tube under argon was charged with FeCl2·4H2O (0.09 mmol, 0.018 g) and formamide (4.5 mmol, 0.20 g), the mixture was heated for 5 min, and then tetrachloromethane (18 mmol, 2.76 g), methanol (4.5 mmol, 0.14 g), and fluorotoluene 1-3 (9 mmol, 1.00 g) (or difluorotoluene 4-6 (9 mmol, 1.15 g)) were added. The sealed tube was placed into an autoclave, and the autoclave was sealed and heated for 6 h at 180 °C. After completion of the reaction, the autoclave was cooled down to room temperature, the tube was opened, and the reaction mixture was filtered through a paper filter. The solvent was distilled off. The target product was separated from the initial mono- or difluorotoluenes by vacuum distillation.
References:
Bayguzina, Alfiya R.;Gallyamova, Leysan I.;Khalilov, Leonard M.;Khusnutdinov, Ravil I. [Journal of Fluorine Chemistry,2019,vol. 226,art. no. 109346] Location in patent:supporting information
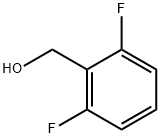
19064-18-7
301 suppliers
$6.00/5g

697-73-4
290 suppliers
$6.00/1g
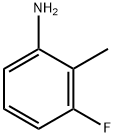
443-86-7
252 suppliers
$10.00/1g

697-73-4
290 suppliers
$6.00/1g

603-83-8
288 suppliers
$11.00/5g

697-73-4
290 suppliers
$6.00/1g