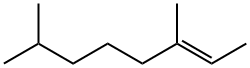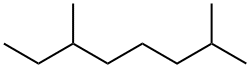
2,6-DIMETHYLOCTANE synthesis
- Product Name:2,6-DIMETHYLOCTANE
- CAS Number:2051-30-1
- Molecular formula:C10H22
- Molecular Weight:142.28
Yield:-
Reaction Conditions:
palladium-carbon;palladium in tetrahydrofuran;
Steps:
13 Chemical Conversion of Geraniol to 2,6-Dimethyloctane Via Direct Hydrogenation
Example 13 Chemical Conversion of Geraniol to 2,6-Dimethyloctane Via Direct Hydrogenation An alternative strategy to convert geraniol to 2,3-dimethyloctane is direct hydrogenation of geraniol to 2,6-dimethyloctane. The initial step in the sequence was the use of palladium on carbon (5.09 g, 5% Pd) to catalyze the hydrogenation of geraniol (184 g, 1.19 mol) in tetrahydrofuran (700 mL) at room temperature for 15 hours. Following the hydrogenation, the reaction mixture was filtered to remove the catalyst, and the filter cake was washed with hexane. The THF and hexane were removed by rotary evaporation. The reaction mixture was separated by vacuum distillation into two cuts; the alcohol product, 3,7-dimethyloctan-1-ol (50.51 g, 0.30 mol) plus a mixture of 3,7-dimethyloct-2-ene and 2,6-dimethyloctane (53.69 g). An ethereal product remained in the stillpot. The isolated octane/octene mixture was reduced with hydrogen gas in the presence of Pd/C (1.69 g, 5% Pd) to yield 2,6-dimethyloctane. The reduction was carried out neat, the only solvent present was a small volume of ethanol used to wash out the flask containing the starting material. The reaction was run in a Parr shaker for 20 hours. Following the hydrogenation the reaction was filtered, and the filter cake was washed with hexane. The crude reaction was concentrated by rotary evaporation. The dimethyloctane was isolated by vacuum distillation (83 to 86° C. at 70 mm Hg.) to yield 40 g (30% yield) of product. The product was identified by both NMR and GC/MS. The yield of the desired 2,6-dimethyl octane can be improved by changing the catalyst and the reaction conditions.
References:
US2011/160501,2011,A1
123-35-3
412 suppliers
$27.00/25g

2051-30-1
56 suppliers
$160.00/1g
3016-19-1
28 suppliers
inquiry

2051-30-1
56 suppliers
$160.00/1g
4057-42-5
6 suppliers
inquiry

2051-30-1
56 suppliers
$160.00/1g

27767-63-1
0 suppliers
inquiry

2051-30-1
56 suppliers
$160.00/1g