
2,9-dichloro-1,10-phenanthroline-5,6-quinone synthesis
- Product Name:2,9-dichloro-1,10-phenanthroline-5,6-quinone
- CAS Number:141622-77-7
- Molecular formula:C12H4Cl2N2O2
- Molecular Weight:279.08

943861-95-8
41 suppliers
inquiry

141622-77-7
10 suppliers
inquiry
Yield:141622-77-7 72%
Reaction Conditions:
with trichlorophosphate for 12 h;Reflux;Inert atmosphere;
Steps:
4.3.3. General procedure III: chlorination of bromo-phts andbromo-phds
Either bromo-pht (5 and 9) or bromo-phd (1e, 2a, 2c) with thesame molar amount of 1.64 mmol was added to phosphoryl chloride (30 mL) to give a red suspension, which was further reuxedfor 12 h under the argon atmosphere with stirring. The mixture wasallowed to cool slightly to the room temperature and the excessphosphoryl chloride was removed by distillation. The yellow res-idue was added cautiously by H2O (20 mL) and subsequent a KOHsolution (0.5 mol/L, 5 mL) in an ice bath until the neutral pH. Thebrown precipitate was ltered, washed with water and dried invacuo. Purication by silica gel column chromatography usingCHCl3 as the eluent gave respective chlorination product (12, 13,14). It is worth pointing out that one polychlorinated product 11could also be isolated in a yield of 53% during the synthesis of 12 byusing 5 as the starting material.
References:
Peng, Yu-Xin;Hu, Bin;Huang, Wei [Tetrahedron,2018,vol. 74,# 34,p. 4495 - 4503]
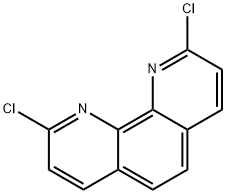
29176-55-4
155 suppliers
$10.00/100mg

141622-77-7
10 suppliers
inquiry
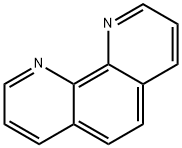
66-71-7
381 suppliers
$6.00/5g

141622-77-7
10 suppliers
inquiry
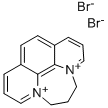
15302-99-5
4 suppliers
inquiry

141622-77-7
10 suppliers
inquiry
![5H-[1,4]Diazepino[1,2,3,4-lmn][1,10]phenanthroline-3,9-dione, 6,7-dihydro-](/CAS/20200611/GIF/39588-59-5.gif)
39588-59-5
0 suppliers
inquiry

141622-77-7
10 suppliers
inquiry