
2-Amino-6-hydroxymethylpyrimidine synthesis
- Product Name:2-Amino-6-hydroxymethylpyrimidine
- CAS Number:2164-67-2
- Molecular formula:C5H7N3O
- Molecular Weight:125.13

2164-66-1
36 suppliers
$155.00/50mg

2164-67-2
63 suppliers
$57.00/100 mg
Yield: 73%
Reaction Conditions:
Stage #1:methyl 2-aminopyrimidine-4-carboxylate with sodium tetrahydroborate in ethanol;dichloromethane for 24 h;
Stage #2: with hydrogenchloride in ethannol;dichloromethane;water;acetone at 0;
Stage #3: with sodium hydrogencarbonate in ethanol;dichloromethane;water;acetone; pH=8
Steps:
4 2-Amino-4-hydroxymethylpyrimidine (Reference Compound No.4-1)
2-Amino-4-methoxycarbonylpyrimidine (3.0g, 20mmol, Reference Compound No.3-1) was suspended in a mixture solvent of ethanol (150mL) and dichloromethane (20mL), then sodium borohydride (2.2g, 59mmol) was added thereto at room temperature, and the whole was stirred for 24 hours. Acetone (20mL) was added gradually under ice-cooling, and then 2M hydrochloric acid was added until the bubbles were no longer formed. Saturated aqueous sodium hydrogencarbonate solution was added to adjust the pH of the reaction mixture to 8, and the precipitated solid was filtered out. The filtrate was concentrated under reduced pressure, then suspended in a 10% methanol-chloroform solution, and the mixture was filtered again with silica gel (5.0g). The filtrate was evaporated under reduced pressure, the precipitated solid was filterd off with ethyl acetate, and dried under reduced pressure to give 1.8g of the title Reference Compound as a pale yellow solid (Yield: 73%) 1H-NMR (400MHz, DMSO-d6) δ 4.30(s,2H),5.35(s,1H),6.48(s,2H),6.65(d,J = 4.9 Hz,1H),8.19(d,J = 4.9 Hz,1H)
References:
SANTEN PHARMACEUTICAL CO., LTD. EP1864977, 2007, A1 Location in patent:Page/Page column 17
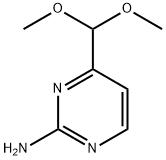
165807-05-6
105 suppliers
$19.00/100mg

2164-67-2
63 suppliers
$57.00/100 mg
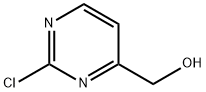
34953-87-2
47 suppliers
inquiry

2164-67-2
63 suppliers
$57.00/100 mg

165807-05-6
105 suppliers
$19.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2164-67-2
63 suppliers
$57.00/100 mg
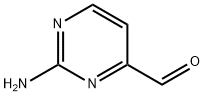
165807-06-7
29 suppliers
inquiry

2164-67-2
63 suppliers
$57.00/100 mg