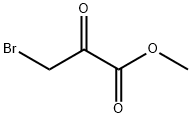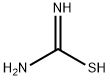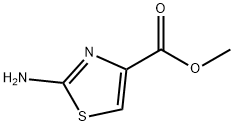
Methyl 2-Aminothiazole-4-carboxylate synthesis
- Product Name:Methyl 2-Aminothiazole-4-carboxylate
- CAS Number:118452-04-3
- Molecular formula:C5H6N2O2S
- Molecular Weight:158.18

5398-36-7
394 suppliers
$6.00/5g
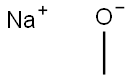
124-41-4
692 suppliers
$12.00/25g

118452-04-3
264 suppliers
$5.00/1g
Yield:118452-04-3 45%
Reaction Conditions:
in methanol at 0; for 1 h;Cooling with ice;
Steps:
3.2
A mixture of 2-amino-thiazole-4-carboxylic acid ethyl ester (38 g, 221 mmol) in methanol (400 mL) was cooled in an ice bath and to it was added 25% sodium methoxide over 30 minutes. The ice bath was removed after 30 minutes. A few small particles were filtered off and to this yellow solution was added saturated aqueous ammonium chloride and it was concentrated to remove excess methanol. The mixture was adjusted to pH=9.0 with sodium bicarbonate and extracted using 1:1 ether/tetrahydrofuran (3×200 mL). The combined organic extracts were washed with water, dried over sodium sulfate and concentrated to give a pale yellow solid which still contained some solvent. It was taken into hexanes, filtered on a 5.5 cm funnel then dried in vacuo to give 2-amino-thiazole-4-carboxylic acid methyl ester as a pale yellow solid (15.6 g, 45%).
References:
US2006/63814,2006,A1 Location in patent:Page/Page column 28

5398-36-7
394 suppliers
$6.00/5g

118452-04-3
264 suppliers
$5.00/1g

67-56-1
746 suppliers
$9.00/25ml
40283-41-8
182 suppliers
$5.00/1g

118452-04-3
264 suppliers
$5.00/1g