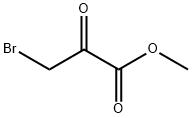
Methyl Bromopyruvate synthesis
- Product Name:Methyl Bromopyruvate
- CAS Number:7425-63-0
- Molecular formula:C4H5BrO3
- Molecular Weight:180.98

67-56-1
737 suppliers
$7.29/5ml-f

1113-59-3
291 suppliers
$14.00/1g

7425-63-0
174 suppliers
$20.00/5g
Yield:-
Reaction Conditions:
hydrogenchloride in water at 30 - 40; for 9 h;
Steps:
2
All starting materials can be obtained from commercial sources, for example. The major components include 3-bromo-2-oxopropionate, ethanol, methanol, concentrated hydrochloric acid (HCl), and sodium carbonate (Na2CO3, or alternatively sodium bicarbonate NaHCO3). Similar to the preparation of Glycolycin, the principal chemical reaction to produce E-Glycolycin or M-Glycolycin is also the esterification of 3-bromo-2-oxopropionate by proper alcohols. Esterification of 3-bromo-2-oxopropionate by ethanol produces E-Glycolycin, whereas esterification of 3-bromo-2-oxopropionate by methanol generates M-Glycolycin. There are at least five specific steps each in the preparation of E-Glycolycin or M-Glycolycin: (1) Place 1 mole of 3-bromo-2-oxopropionate solid (167 grams) in each reaction chamber; (2) Add 2 moles of pure ethanol (for E-Glycolycin preparation) or methanol (for M-Glycolycin preparation) to the respective reaction chamber; the excess molar amount of alcohols favors the chemical reaction toward fully utilization of 3-bromo-2-oxopropionate with favorable yield of E-Glycolycin of M- Glycolycin; (3) Add a small amount of concentrated hydrochloric acid to each chamber as the catalyst for the esterification reaction, heat the chamber to 4O0C, and let the reaction continue under constant stirring. After 60 min, cool to 3O0C, and let the reaction continue for additional 8 hours with constant stirring; (4) After the reaction is completed, cool the reaction products to 0°C with an ice-bath, add solid Na2CO3 (or NaHCO3) in the amount only sufficient to neutralize the added HCl. Do not use excess Na2CO3 (or NaHCO3) or add water, since excess Na2CO3 (or NaHCO3) or the presence of water facilitates hydrolysis of the ester products; (5) Evaporate off the excess alcohol under low pressure with proper vacuum but without heating. Alternatively, E- Glycolycin stored with the excess ethanol -2O0C.
References:
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM WO2006/20403, 2006, A2 Location in patent:Page/Page column 58

600-22-6
366 suppliers
$6.00/5g

7425-63-0
174 suppliers
$20.00/5g
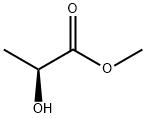
27871-49-4
271 suppliers
$8.00/25g

7425-63-0
174 suppliers
$20.00/5g