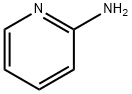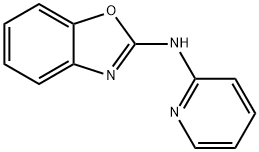
2-BenzoxazolaMine, N-2-pyridinyl- synthesis
- Product Name:2-BenzoxazolaMine, N-2-pyridinyl-
- CAS Number:6458-60-2
- Molecular formula:C12H9N3O
- Molecular Weight:211.22
Yield:6458-60-2 60%
Reaction Conditions:
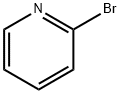
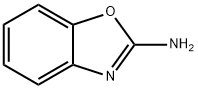
Stage #1: 2-bromo-pyridine;1,3-benzoxazol-2-aminewith caesium carbonate;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1,4-dioxane; for 0.166667 h;Inert atmosphere;
Stage #2: with tris-(dibenzylideneacetone)dipalladium(0) in 1,4-dioxane at 90; for 30 h;
Steps:
K
2-Bromopyridine (1) (lOg, 6.3mmol), benzo[d]oxazol-2-amine (2) (0.871g,6.4mmol), Xantphos (0.366g, 0.63mmol), and Cs2003 (3.09g, 9.4mmol) were combined in dry 1,4-dioxane (l5mL). The reaction mixture was degassed with N2(g) and placed under vacuum for 10mm. Pd2(dba)3 (0.289g, 0.31 mmol) was then added and the resulting reaction mixture was heated at 90°C for 30h. It wasthen poured onto demineralized water (200mL), and extracted with EtOAc (3 x lOOmL). The organic phases were combined, dried over Na2SO4, filtered and subsequently evaporated under vacuum. The resulting residue was purified by flash chromatography, eluting with EtOAc/Hexane (1:1) to provide N-(pyridin-2- yl)benzo[d]oxazol-2-amine (3) as a yellow solid (0.8g, 60%).LCMS (ES): Found 212.1 [MH]+.
References:
WO2014/72714,2014,A1 Location in patent:Page/Page column 35