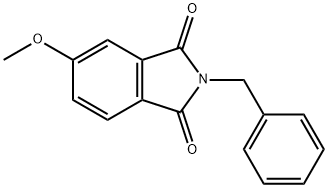
2-benzyl-5-methoxyphthalimide synthesis
- Product Name:2-benzyl-5-methoxyphthalimide
- CAS Number:1007455-12-0
- Molecular formula:C16H13NO3
- Molecular Weight:267.28
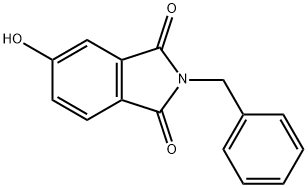
3997-85-1
1 suppliers
inquiry

74-88-4
343 suppliers
$15.00/10g

1007455-12-0
12 suppliers
$45.00/10mg
Yield:1007455-12-0 96%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 19 - 24;
Steps:
2-Benzyl-5-methoxy-isoindole-1 ,3-dione lodomethane (168.51 g, 73.9 ml_, 1.187 mol, 1.5 equiv) was added slowly to stirred suspension of 2-Benzyl-5-hydroxy-isoindole-1 ,3-dione (200.2 g, 0.791 mol, 1.0 equiv) and K2CO3 (163.81 g, 1.187 mol, 1.5 equiv) in DMF (2.5 L). The reaction mixture warmed from 19 to 24 0C during the addition. The yellow suspension was stirred at room temperature overnight. The reaction mixture was poured into H2O (7 L). The resulting white solid was collected, washed with H2O (4 x 1 L), dried on the filter under a stream of N2 for 2 h, then in a vacuum oven at -55-60 0C for 2 h to give 202.02 g (96%) of 2-Benzyl-5-methoxy-isoindole- 1 ,3-dione. MS: APCI: M+: 267.0 (267.1 ).
References:
WO2008/20306,2008,A2 Location in patent:Page/Page column 22-23
28281-76-7
103 suppliers
$11.00/100mg

100-46-9
461 suppliers
$5.00/5G

1007455-12-0
12 suppliers
$45.00/10mg
22895-19-8
18 suppliers
$297.00/10ml-f

100-46-9
461 suppliers
$5.00/5G

1007455-12-0
12 suppliers
$45.00/10mg