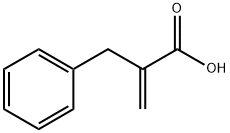
2-Benzylacrylic acid synthesis
- Product Name:2-Benzylacrylic acid
- CAS Number:5669-19-2
- Molecular formula:C10H10O2
- Molecular Weight:162.19
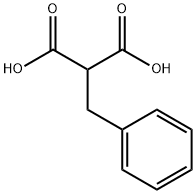
616-75-1
134 suppliers
$45.00/5 g

5669-19-2
306 suppliers
$14.00/5g
Yield:5669-19-2 90%
Reaction Conditions:
Stage #1:2-benzylmalonic acid with formaldehyd;diethylamine in ethyl acetate at 0 - 20; for 1.5 h;Heating / reflux;
Stage #2: with hydrogenchloride;water at 10;
Steps:
3; 4 2(S)-Benzyl-3-[5-(3-ethylamino-phenyl)-9-methyl-2-aza-bicyclo[3.3.1]non-2-yl]propan-1-ol (17, Example 3) and 2R-Benzyl-3-[5-(3-ethylamino-phenyl)-9-methyl-2-aza-bicyclo[3.3.1]non-2-yl]propan-1-ol (18, Example 4)
A solution of benzylmalonic acid (20.0 g, 0.103 mol, 1 eq) and paraformaldehyde (4.94 g, 0.164 mol, 1.6 eq) in ethyl acetate (150 mL) was cooled (0° C.) and treated with diethylamine (10.65 mL, 0.103 mol, 1 eq) drop wise, keeping the reaction temperature below 20° C. The reaction was then warmed to reflux for 90 minutes and cooled again on ice. The homogeneous solution was treated with water (20 mL) and concentrated aqueous HCl (12N) (9.0 mL, 0.108 mol) drop wise, keeping the reaction temperature below 10° C. The phases were then separated. The organic layer was washed with brine (100 mL), dried over sodium sulfate, filtered, and the filtrate concentrated under vacuum giving 2-methylene-3-phenylpropanoic acid (10) as a white solid (15 g, 90%). A solution of 10 (10.3 g, 63.5 mmol) in methanol (100 mL) was cooled on ice and treated dropwise with thionyl chloride (14.0 mL, 192 mmol). After warming to ambient temperature, the reaction was stirred for 18 hours at room temperature and concentrated in vacuo. The crude product was dissolved in diethyl ether, washed with a saturated aqueous solution of sodium bicarbonate and brine, dried over sodium sulfate, filtered, and the filtrate concentrated in vacuo providing the methyl ester 11 (11.2 g, 100%) as a pale yellow oil. To a solution of (+/-)2 (2 g, 8.643 mmol, 1 eq), 2-benzyl-acrylic methyl ester 11 (3.19 g, 0.0182 mol, 2.1 eq) in methanol (25 mL) was added triethylamine (3.54 mL, 25.93 mmol, 3 eq) and the solution was heated to reflux for 16 hours under argon. The reaction mixture was concentrated in vacuo, then dissolved in ethyl acetate (200 mL). The mixture was extracted with a 1N aqueous solution of hydrochloric acid (2×200 mL). The combined aqueous phase was alkalinized to pH 8 with solid sodium hydrogenocarbonate, then extracted with ethyl acetate (3×150 mL). The combined organic extracts were dried (sodium sulfate), filtered and concentrated in vacuo to afford a clear tan oil. This oil was purified by column chromatography (eluent:dichloromethane/methanol=98:2) affording the desired compound 12 (mixture of diastereoisomers) (1.07 g, 30.5%). To a stirred solution of 12 (0.265 g, 0.650 mmol, 1 eq) in anhydrous dichloromethane (3 mL) was added triethylamine (154 μL, 11.05 mmol, 1.7 eq), followed by drop wise addition of a solution of N-phenyltrifluoromethanesulfonimide (0.347 g, 0.975 mmol, 1.5 eq) in anhydrous dichloromethane (0.5 mL) at room temperature under argon. The reaction mixture was allowed to stir for 1 h at room temperature. Dichloromethane (20 mL) was added to the mixture, which was washed with a IN aqueous solution of sodium hydroxide (20 mL) and brine (20 mL). The organic layer was then dried (sodium sulfate), filtered, and concentrated in vacuo. The crude product was purified by column chromatography (eluent:dichloromethane) affording the desired compound 13 (mixture of diastereoisomers) (0.281 g, 80%). A mixture of triflate 13 (0.500 g, 0.927 mmol, 1 eq), diphenylphosphinoferrocene (DPPF) (77 mg, 15 mol %, 0.139 mmol), Pd2(dba)3 (42.5 mg, 5 mol %), sodium tert-butoxide (0.196 g, 2.04 mmol, 2.2 eq) and benzophenone imine (187 μL, 1.112 mmol, 1.2 eq) in anhydrous toluene (40 mL) was degassed using vacuum and argon. The mixture was then heated to 80° C. for 16 hours. The reaction mixture was cooled to room temperature and quenched by addition of an aqueous saturated solution of ammonium chloride (100 mL). The organic layer was separated. The aqueous layer was further extracted with dichloromethane (2×100 mL) and the combined organic extracts were washed with brine (100 mL) prior to drying with sodium sulfate, filtering, and concentration in vacuo. The crude imine 14 was used for the next step without further purification (0.460 g, 85%). A mixture of the crude imine 14 (0.4 g, 0.682 mmol, 1 eq), hydroxylamine hydrochloride (96.5 mg, 1.365 mmol, 2 eq), sodium acetate (282 mg, 3.425 mmol, 5 eq) in methanol (10 mL) was stirred at room temperature for 45 minutes. The reaction mixture was concentrated in vacuo and partitioned between dichloromethane (3×50 mL) and water (50 mL). The combined organic extracts were washed with brine (50 mL) and dried over sodium sulfate. The organic extracts were filtered and concentrated in vacuo. The crude product was purified by column chromatography (eluent:dichloromethane/methanol=99:1) affording the desired compound 15 (mixture of diastereoisomers) (0.295g, 100%). To a cooled (0° C.), stirred solution of the aniline derivative 15 (0.200 g, 0.492 mmol, 1 eq) and triethylamine (135μL, 0.983 mmol, 2eq) in anhydrous dichloromethane (10 mL) was added drop wise acetyl chloride (55 μL, 0.738 mmol, 1.5 eq). The mixture was stirred for 1 hour at room temperature and washed with an aqueous saturated solution of sodium hydrogenocarbonate. The aqueous layer was further extracted with dichloromethane (2×25 mL). The combined organic extracts were washed with brine (50 mL), then dried (sodium sulfate) prior to filtration and concentration in vacuo. The crude product was purified by column chromatography (eluent:dichloromethane/methanol=99.5:0.5) affording the desired compound 16 (mixture of diastereoisomers) (0.136 g, 69%). The acetamide 16 (0.130 g, 0.289 mmol, 1 eq) was dissolved in anhydrous tetrahydrofuran. The solution was cooled (0° C.) and treated with a solution of borane in tetrahydrofuran (0.7225 mmol, 2.5 eq). The reaction mixture was refluxed for 2 hours. The reaction mixture was cooled to room temperature and then quenched by addition of methanol (1 mL). The mixture was heated to reflux for 15 min, cooled to room temperature and partitioned between dichloromethane (3×25 mL) and an aqueous saturated solution of sodium hydrogenocarbonate (25 mL). The combined organic layers were further washed with brine (25 mL) prior to drying (sodium sulfate), filtration and concentration in vacuo. The crude product was purified by column chromatography (eluent:dichloromethane/methanol=98:2) affording the desired compounds 17 (Example 3) (38 mg, 32%) ([M+H]+ 407) and 18 (Example 4) (24 mg, 20%) ([M+H]+ 407).
References:
Adolor Corporation US2005/203123, 2005, A1 Location in patent:Page/Page column 25-26

20593-63-9
5 suppliers
inquiry

5669-19-2
306 suppliers
$14.00/5g

50-00-0
850 suppliers
$10.00/25g

616-75-1
134 suppliers
$45.00/5 g

5669-19-2
306 suppliers
$14.00/5g
2495-35-4
272 suppliers
$5.00/1g

5669-19-2
306 suppliers
$14.00/5g
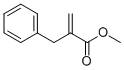
3070-71-1
71 suppliers
$77.50/250 mg

5669-19-2
306 suppliers
$14.00/5g