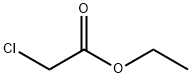
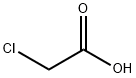
2-BENZYLAMINO-THIAZOL-4-ONE synthesis
- Product Name:2-BENZYLAMINO-THIAZOL-4-ONE
- CAS Number:17385-69-2
- Molecular formula:C10H10N2OS
- Molecular Weight:0
Yield:17385-69-2 359 mg
Reaction Conditions:
with sodium acetate in ethanol; for 6 h;Reflux;Inert atmosphere;
Steps:
2 10.2.3 Preparation of compound 21a
General procedure: To a magnetically stirred suspension of phenylthiourea (19a, 304 mg, 2 mmol) and anhydrous NaOAc (820 mg, 10 mmol) in absolute ethanol (7 mL) was added ethyl chloroacetate (20, 0.5 mL, 4 mmol) and the mixture was heated under reflux for 6 h. The reaction mixture was concentrated under reduced pressure. The residue was diluted with water (50 mL) and extracted with EtOAc (3 × 15 mL). The combined EtOAc extracts were dried (anhydrous Na2SO4), filtered and the filtrate was concentrated under rotary vacuum evaporator to obtain a solid mass which was recrystallized from EtOAc to obtain the desired product as white solid. 10.2.3.2
2-(Benzylimino)thiazolidin-4-one (21b)
white solid; 359 mg, 87% yield, 1H NMR (400 MHz, CD3SOCD3) δ = 7.37-7.25 (m, 5H), 4.61 (s, 2H), 3.90 (s, 2H); 13C NMR (100 MHz, CD3SOCD3): δ = 187.62, 182.69, 138.03, 137.57, 129.00, 128.94, 128.72, 128.18, 127.99, 127.95, 127.82, 127.79, 48.31.
References:
Arfeen, Minhajul;Bhagat, Shweta;Patel, Rahul;Prasad, Shivcharan;Roy, Ipsita;Chakraborti, Asit K.;Bharatam, Prasad V. [European Journal of Medicinal Chemistry,2016,vol. 121,p. 727 - 736] Location in patent:supporting information
622-78-6
238 suppliers
$12.00/1g

17385-69-2
8 suppliers
inquiry

100-46-9
461 suppliers
$5.00/5G

17385-69-2
8 suppliers
inquiry

20949-66-0
21 suppliers
$221.00/500mg

100-46-9
461 suppliers
$5.00/5G

17385-69-2
8 suppliers
inquiry