
2-(benzyloxy)-5-chlorobenzoic acid synthesis
- Product Name:2-(benzyloxy)-5-chlorobenzoic acid
- CAS Number:52803-75-5
- Molecular formula:C14H11ClO3
- Molecular Weight:262.69
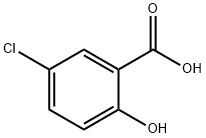
321-14-2
367 suppliers
$15.00/25g

100-44-7
628 suppliers
$13.50/250G

52803-75-5
12 suppliers
$57.50/250 mg
Yield: 90.3%
Reaction Conditions:
Stage #1:5-chloro-2-hydroxybenzoic acid;benzyl chloride with potassium carbonate in N,N-dimethyl-formamide at 20;
Stage #2: with sodium hydroxide in tetrahydrofuran;methanol for 12 h;
Steps:
4.2.5. Synthesis of 2-(benzyloxy)-5-chlorobenzoic acid (7)
A solution of 5-chloro-2-hydroxybenzoic acid (2) (2.0 g,11.6 mmol) in dry DMF (15 mL) was added K2CO3 (4.81 g,34.8 mmol) portionwise, followed by benzyl chloride (1.33 mL,34.8 mmol) at room temperature. The mixture was stirred at roomtemperature and monitored by thin layer chromatography (TLC).The resulting mixture was quenched with saturated NH4Cl solution(10 mL), and the mixturewas extracted with AcOEt (3 10 mL). Theorganic layers were washed by brine, dried over MgSO4 andconcentrated at reduced pressure. The residue was dissolved in asolution of THF-EtOH (1:1, 10 mL), 5 moL/L NaOH (5.0 mL) was thenadded slowly. The reaction solution was stirred for 12 h andconcentrated at reduced pressure. The aqueous layer was thenacidified with 10% HCl to pH 2e3. The precipitated product wasfiltered and washed with water (20 mL) to yield the desired compound7 (2.74 g, 90.3%) as a white powder.
References:
Chen, Xiaojing;Wang, Guangbao;Mohammed Alsayed, Ali Mohammed;Du, Zongxuan;Lu liu;Ma, Yue;Liu, Peng;Zhang, Qianwen;Chen, Xianxin;Chen, Wenbin;Ye, Faqing;Zheng, Xiaohui;Liu, Zhiguo [European Journal of Medicinal Chemistry,2021,vol. 214]

103620-87-7
5 suppliers
inquiry

52803-75-5
12 suppliers
$57.50/250 mg

321-14-2
367 suppliers
$15.00/25g

100-39-0
423 suppliers
$10.00/10 g

52803-75-5
12 suppliers
$57.50/250 mg
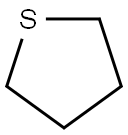
110-01-0
201 suppliers
$10.00/5g

321-14-2
367 suppliers
$15.00/25g

100-44-7
628 suppliers
$13.50/250G

52803-75-5
12 suppliers
$57.50/250 mg

321-14-2
367 suppliers
$15.00/25g

52803-75-5
12 suppliers
$57.50/250 mg