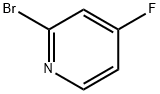
2-Bromo-4-fluoropyridine synthesis
- Product Name:2-Bromo-4-fluoropyridine
- CAS Number:357927-50-5
- Molecular formula:C5H3BrFN
- Molecular Weight:175.99
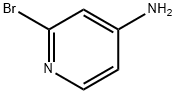
7598-35-8
367 suppliers
$6.00/250mg

357927-50-5
221 suppliers
$11.00/1g
Yield:-
Reaction Conditions:
Stage #1:2-bromo-4-aminopyridine with tetrafluoroboric acid;sodium nitrite in water at 0 - 20; for 12.0833 h;
Stage #2: with sodium hydrogencarbonate in water
Steps:
5.1
Example 5N-(4-{[l-(4-fluoropyridin-2-yl)-lH-indol-5-yl]oxy}pyrimidin-2-yl)benzene-l,3-diamine (Compound 76)For the preparation of Compound 77, 2-bromo-4-fluoropyridine was prepared utilizing a modified procedure described in the reference below, and used in the indole arylation reaction. Thibault, C; L'ηeureux, A.; Bhide, R. S.; Ruel, R. Org. Lett. 2003, 5, 5023-5025.Step 1: 2-bromo-4-fluoropyridine.To a stirred suspension of 2-bromopyridin-4-amine (0.50 g, 2.9 mMol) in 50% aqueous HBF4 (6.0 mL) was added a solution of sodium nitrite (0.24 g, 3.5 mMol) in water (3.0 mL) over 5 minutes at 0 0C. The resulting mixture was allowed to warm to ambient, and was stirred for 12 hours. Solid NaHCO3 was added slowly, until CO2 evolution ceased, and the resulting aqueous solution was extracted with EtOAc (2 x 30 mL). The combined organic extracts were washed with saturated NaHCO3 (1 x 30 mL), and brine (1 x 30 mL). The organic layer was dried over magnesium sulfate, filtered and concentrated to afford the title compound.
References:
MERCK & CO., INC. WO2007/35309, 2007, A1 Location in patent:Page/Page column 63