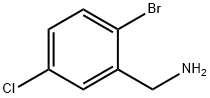
(2-bromo-5-chlorophenyl)methanamine synthesis
- Product Name:(2-bromo-5-chlorophenyl)methanamine
- CAS Number:942400-60-4
- Molecular formula:C7H7BrClN
- Molecular Weight:220.49
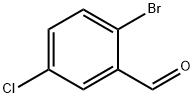
174265-12-4
223 suppliers
$6.00/1g

942400-60-4
16 suppliers
inquiry
Yield:942400-60-4 95%
Reaction Conditions:
Stage #1: 2-bromo-5-chlorobenzaldehydewith hydroxylamine in methanol at 20; for 4 h;
Stage #2: with acetic acid;zinc
Steps:
154
PREPARATION 154 (2-Bromo-5-chlorophenyl)methanamine 2-Bromo-5-chlorobenzaldehyde (2 g, 9.1 1 mmol) was dissolved in 100 ml methanol. Hydroxylamine (2.2 ml, 36.5 mmol) was added and the mixture was stirred at room temperature for 4 h. The mixture was evaporated under reduced pressure and the mixture was partitioned between ethyl acetate and water. The organic layer was washed with water, dried over sodium sulphate, filtered and evaporated under reduced pressure. The solid residue was dissolved in 60 ml acetic acid. Zinc dust (2.40 g, 36.7 mmol) was added and the suspension was stirred overnight. The mixture was filtered through Celite. The filtrate was evaporated and the residue re-evaporated twice from cyclohexane. The semi-solid residue was triturated with ether to give a solid which was collected by filtration, washed with ether and dried in the oven to give 2 g (9.07 mmol, 95%) of the title compound as a white solid. Purity 95%. 1 H NMR (400 MHz, DMSO-d6) δ ppm 1 .90 (s, 2 H), 3.73 (s, 2 H), 7.25 (dd, J=8.60, 2.74 Hz, 1 H), 7.58 (d, J=8.60 Hz, 1 H), 7.63 (d, J=2.74 Hz, 1 H). UPLC/MS (3 min) retention time 0.75 min. LRMS: m/z 220, 222 (M+1 ).
References:
WO2013/10880,2013,A1 Location in patent:Page/Page column 156-157
![1H-Isoindole-1,3(2H)-dione, 2-[(2-bromo-5-chlorophenyl)methyl]-](/CAS/20210305/GIF/1338254-51-5.gif)
1338254-51-5
2 suppliers
inquiry

942400-60-4
16 suppliers
inquiry
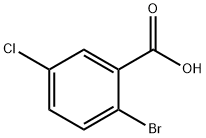
21739-93-5
251 suppliers
$5.00/1g

942400-60-4
16 suppliers
inquiry
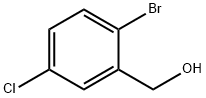
60666-70-8
92 suppliers
$9.00/1g

942400-60-4
16 suppliers
inquiry
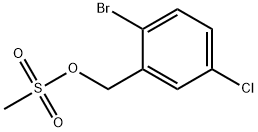
1338254-50-4
2 suppliers
inquiry

942400-60-4
16 suppliers
inquiry