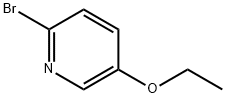
2-BROMO-5-ETHOXYPYRIDINE synthesis
- Product Name:2-BROMO-5-ETHOXYPYRIDINE
- CAS Number:42834-01-5
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05

55717-45-8
278 suppliers
$5.00/250mg

75-03-6
354 suppliers
$11.00/5g

42834-01-5
40 suppliers
$80.00/1g
Yield:42834-01-5 100%
Reaction Conditions:
Stage #1: 6-bromo-pyridin-3-olwith sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: ethyl iodide in N,N-dimethyl-formamide at 20; for 3.5 h;
Steps:
12.1
Reference Example 12 [0441] Step 1 [0442] To a solution of Compound vii-2 (2.0g, 11.49mmol) in DMF (20mL) was added sodium hydride (0.644g, 16.09mmol) at room temperature, and the mixture was stirred for 30 minutes. Iodoethane (1.858mL, 22.99mmol) was added to the mixture and the resulting mixture was stirred for additional 3.5 hours at room temperature. To the reaction mixture was added water, and the mixture was extracted with ethyl acetate. The organic layer was washed by water and brine, dried over anhydrous magnesium sulphate, and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexane-ethyl acetate) to give Compound vii-3 (2.32g, Yield 100%). [0443] 1H-NMR (CDCl3) δ: 8.03 (1H, d, J = 2.75 Hz), 7.35 (1H, d, J = 8.69 Hz), 7.07 (1H, dd, J = 8.62, 2.97 Hz), 4.05 (2H, q, J = 6.91 Hz), 1.43 (3H, t, J = 6.94 Hz).
References:
EP2752410,2014,A1 Location in patent:Paragraph 0441-0443

55717-45-8
278 suppliers
$5.00/250mg

74-96-4
430 suppliers
$9.00/5g

42834-01-5
40 suppliers
$80.00/1g
89943-11-3
54 suppliers
$65.00/100mg

42834-01-5
40 suppliers
$80.00/1g