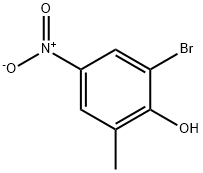
2-Bromo-6-methyl-4-nitrophenol synthesis
- Product Name:2-Bromo-6-methyl-4-nitrophenol
- CAS Number:4186-49-6
- Molecular formula:C7H6BrNO3
- Molecular Weight:232.03

99-53-6
167 suppliers
$9.00/5g

4186-49-6
27 suppliers
inquiry
Yield:4186-49-6 80%
Reaction Conditions:
with bromine;acetic acid at 20; for 4 h;
Steps:
10 j0800] Preparation of 2-bromo-6-methyl-4-nitrophenol:
Br2 (122.2 g, 0.765 mol) was added into a solution of 2-methyl-4-nitrophenol (90.0 g, 0.588 mol) in HOAc (1.17 L) at room temperature.
The resulting solution was stirred at room temperature for 4 h. TLC showed the reaction was complete.
The solution was added into ice-water (3 L) slowly and filtered.
The filter cake was dissolved into EA (2.5 L) and washed with saturated NaHSO3 (3*500 mL).
The EtOAc layer was dried over anhydrous Na2SO4, filtered and concentrated in vacuo to afford 2-bromo-6-methyl-4-nitrophenol (110 g, 80%) as yellow solid. 1H-NMR: 400 MHz, (CDCl3) δ: 8.30 (d, J=2.0 Hz, 1H), 8.05 (s, 1H), 6.22 (s, broad, 1H), 2.41 (s, 3H).
References:
US2013/281433,2013,A1 Location in patent:Paragraph 0800
609-22-3
59 suppliers
$8.00/500mg

4186-49-6
27 suppliers
inquiry
20294-50-2
65 suppliers
$12.00/250mg
95-48-7
431 suppliers
$19.00/25g

4186-49-6
27 suppliers
inquiry

609-22-3
59 suppliers
$8.00/500mg

4186-49-6
27 suppliers
inquiry
13319-71-6
131 suppliers
$12.00/250mg

4186-49-6
27 suppliers
inquiry