
2-Bromo-6-nitro-4-trifluoromethoxyaniline synthesis
- Product Name:2-Bromo-6-nitro-4-trifluoromethoxyaniline
- CAS Number:886499-21-4
- Molecular formula:C7H4BrF3N2O3
- Molecular Weight:301.02

886499-21-4
62 suppliers
$38.00/5g
1326311-36-7
0 suppliers
inquiry
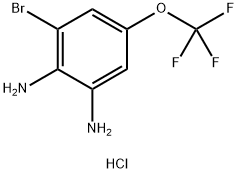
Yield:1326311-36-7 41%
Reaction Conditions:
Stage #1: {2-bromo-6-nitro-4-[(trifluoromethyl)oxy]phenyl}aminewith sodium dithionite in ethanol;water at 20;
Stage #2: with hydrogenchloride in 1,4-dioxane;diethyl ether;
Steps:
78.A
Step A{2-amino-3-bromo-5-[(trifluoromethyl)oxy]p enyl}amine[00345] A solution of {2-bromo-6-nitro-4-[(trifluoromethyl)oxy]phenyl}amine (5.00 g, 16.6 mmol, Matrix Scientific) in ethanol (100 mL) was treated with a solution of sodium dithionite (11.6 g, 66.4 mmol) in water (50 mL) and allowed to stir at rt overnight. A second portion of sodium dithionite (5.80 g, 33.2 mmol) was added as a solution in water and the reaction was stirred for an additional 2 h. The reaction mixture was concentrated to remove some of the ethanol, then diluted with water and extracted with ethyl acetate (2x). The combined organic layers were washed with saturated aqueous ammonium chloride (2x), 10% aqueous sodium carbonate (1x) and brine (1x). After drying over sodium sulfate, the solution was concentrated to a brown oil. This was slurried in diethyl ether and treated with 4 N HCI in dioxane. The mixture was filtered and the solids rinsed with diethyl ether to afford the title compound as the HCI salt (2.08 g, 41 %).
References:
WO2011/97491,2011,A1 Location in patent:Page/Page column 175