2-bromo-6-phenoxypyridine synthesis
- Product Name:2-bromo-6-phenoxypyridine
- CAS Number:83247-00-1
- Molecular formula:C11H8BrNO
- Molecular Weight:250.09
Yield: 93%
Reaction Conditions:
with potassium tert-butylate in dimethyl sulfoxide at 160; for 7 h;
Steps:
16
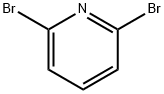
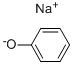
Preparation Example 16. C-(6-Phenoxy-pyridin-2-yl)-methylamine To a solution of 2,6-dibromopyridine (20g, 84.4mmol) and phenol (7.94g, 84.4mmol) in dimethylsulfoxide (200mL) was added potassium tert-butoxide (9.47g, 84.4mmol), and the solution was stirred at 160°C for 7 hours. The reaction solution was allowed to room temperature, ethyl acetate and water were added for partitioning, the organic layer was washed with water and dried over anhydrous magnesium sulfate. The solvent was evaporated, the residue was purified by silica gel column chromatography (hexane : ethyl acetate = 10 : 1), and 2-bromo-6-phenoxy-pyridine (19.6g, 93%) was obtained as a yellow solid. Next, to a solution of the resulting 2-bromo-6-phenoxy-pyridine (1.0g, 4.0mmol) in N,N-dimethylformamide (30mL) were added zinc cyanide (940mg, 8.0mmol) and tetrakis(triphenylphosphine)palladium(0) (924mg, 0.8mmol) under nitrogen atmosphere, and the mixture was stirred at 100°C for 1 hour. The reaction mixture was allowed to room temperature, ethyl acetate and water were added for partitioning, the organic layer was washed with water and dried over anhydrous magnesium sulfate. The solvent was evaporated, the residue was purified by silica gel column chromatography (hexane : ethyl acetate = 10 : 1), and 6-phenoxy-pyridine-2-carbonitrile (524mg, 67%) was obtained as a white solid. 10% Palladium-carbon (50mg) was added to a solution of the resulting 6-phenoxy-pyridine-2-carbonitrile (100mg, 0.51mmol) in methanol (5.0mL), the mixture was stirred at room temperature for 24 hours under hydrogen atmosphere (1 atm). The catalyst was removed by filtration, and the filtrate was concentrated to obtain the title compound (65mg, 64%) as a colorless oil.
References:
Eisai R&D Management Co., Ltd. EP1782811, 2007, A1 Location in patent:Page/Page column 70-71