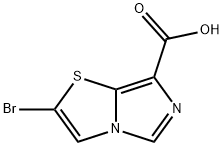
2-BroMoiMidazo[5,1-b]thiazole-7-carboxylic acid synthesis
- Product Name:2-BroMoiMidazo[5,1-b]thiazole-7-carboxylic acid
- CAS Number:901122-45-0
- Molecular formula:C6H3BrN2O2S
- Molecular Weight:247.07
![2-Bromo-Imidazo[5,1-B]Thiazole-7-Carboxylic Acid Ethyl Ester](/CAS/20180629/GIF/901122-44-9.gif)
901122-44-9
22 suppliers
inquiry
![2-BroMoiMidazo[5,1-b]thiazole-7-carboxylic acid](/CAS/GIF/901122-45-0.gif)
901122-45-0
8 suppliers
inquiry
Yield:901122-45-0 100%
Reaction Conditions:
Stage #1: ethyl 2-bromoimidazo[5,1-b]thiazole-7-carboxylatewith potassium hydroxide;water in methanol at 20; for 18 h;
Stage #2: with hydrogenchloride in water at 0;Product distribution / selectivity;
Steps:
6
Example 6; A 2 M aqueous solution (10 ml) of potassium hydroxide was added to a solution of ethyl 2-bromoimidazo[5,1-b]thiazole-7-carboxylate (265 mg, 0.96 mmol) in methanol (20 ml), and the mixture was stirred at room temperature for 18 h. Methanol was removed by distillation under reduced pressure, and the reaction solution was poured into 2 M hydrochloric acid (30 ml) under ice cooling to precipitate a solid. The resultant solid was washed with water, and a mixed solvent composed mainly of ethanol and toluene was added thereto. The solvent was removed by distillation under reduced pressure, and the residue was thoroughly dried to give 2-bromoimidazo[5,1-b]thiazole-7-carboxylic acid (236 mg, quantitative) as a white solid.
References:
EP1840129,2007,A1 Location in patent:Page/Page column 13