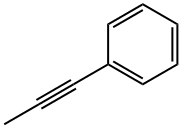
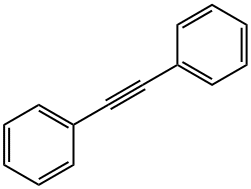
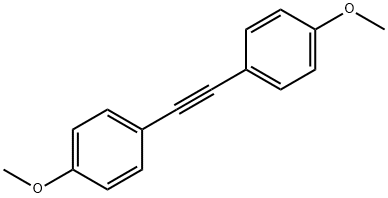
2-Butyne synthesis
- Product Name:2-Butyne
- CAS Number:503-17-3
- Molecular formula:C4H6
- Molecular Weight:54.09
Yield:501-65-5 99%
Reaction Conditions:
with Mo(≡N)(OSiPh3)3(phen);manganese(ll) chloride in toluene at 80; for 3.5 h;Schlenk technique;Inert atmosphere;Molecular sieve;Reagent/catalyst;
Steps:
I.11 11. Alkyne Metathesis by Activation of [Mo(≡N)(OSiPh3)3(Phen)] by Means of MnCl2 Prior to the Addition of the Substrate
11. Alkyne Metathesis by Activation of [Mo(≡N)(OSiPh3)3(Phen)] by Means of MnCl2 Prior to the Addition of the Substrate
In a dry 25 mL Schlenk-tube, which has been flooded with argon, [Mo(≡N)(OSiPh3)3(phen)].0.5 toluene (0.100 mmol, 116.2 mg), MnCl2 (0.100 mmol, 12.6 mg) and molecular sieve (1.00 g, 5Å, powder) were suspended in 5 mL of toluene and the mixture was heated to 80° C. After 30 min 1-penyl-1-propine (1.00 mmol, 116.2 mg) were added, the reaction mixture was stirred for 3 h at 80° C. and was filtered after cooling over a short silica gel column. The filtrate was concentrated and the residue was purified by column chromatography (hexane). 88.2 mg of tolane (99%) were isolated as white solid, the spectroscopic and analytical data of which were identical to those of a commercial sample. M.p.=59-61° C
References:
Studiengesellschaft Kohle mbH;Fuerstner, Alois;Heppekausen, Johannes;Hickmann, Volker;Stade, Robert US8993470, 2015, B2 Location in patent:Page/Page column 36; 37; 47; 48
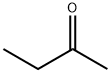
78-93-3
6 suppliers
$30.80/500ml
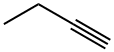
107-00-6
72 suppliers
$85.00/25 g

503-17-3
69 suppliers
$35.00/5g
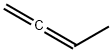
590-19-2
56 suppliers
$225.00/5 g
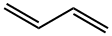
106-99-0
240 suppliers
$77.00/100mL