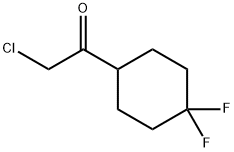
2-chloro-1-(4,4-difluorocyclohexyl)ethanone synthesis
- Product Name:2-chloro-1-(4,4-difluorocyclohexyl)ethanone
- CAS Number:1037834-16-4
- Molecular formula:C8H11ClF2O
- Molecular Weight:196.62

376348-75-3
26 suppliers
$90.00/50mg
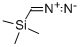
18107-18-1
208 suppliers
$33.00/5g

1037834-16-4
5 suppliers
inquiry
Yield:1037834-16-4 85%
Reaction Conditions:
Stage #1: 4,4-difluorocyclohexane-1-carbonyl chloride;diazomethyl-trimethyl-silanewith triethylamine in diethyl ether at -25 - 20; for 5 h;
Stage #2: with hydrogenchloride in diethyl ether at -20 - 20; for 5 h;
Steps:
Preparation of 2-chloro-1-(4,4-difluorocyclohexyl)ethanone; To a solution containing trimethylsilyldiazomethane in ether (1.70 g, 15.0 mmol 7.50 mL) and triethylamine (15.0 mmol) at -25 °C, was dropwise added a solution of commercially available cyclohexanecarboxylic acid chloride (1.00 g, 6.80 mmol) in anhydrous ether (25 mL). While agitated the reaction mixture was allowed to warm to room temperature
References:
WO2008/84300,2008,A1 Location in patent:Page/Page column 39-40
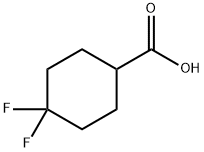
122665-97-8
280 suppliers
$18.00/250mg

1037834-16-4
5 suppliers
inquiry