2-Chloro-2'-methylbenzophenone synthesis
- Product Name:2-Chloro-2'-methylbenzophenone
- CAS Number:4888-03-3
- Molecular formula:C14H11ClO
- Molecular Weight:230.69
Yield:4888-03-3 92.5%
Reaction Conditions:
Stage #1: 2-methylchlorobenzenewith iodine;magnesium in diethyl ether; for 4 h;Inert atmosphere;
Stage #2: o-chlorobenzoyl chloride in 5,5-dimethyl-1,3-cyclohexadiene;diethyl ether; for 36 h;Cooling with ice;Reflux;
Steps:
2.1; 2.2 (1) Preparation of format reagent
48 g (2.0 M) of magnesium powder was taken, placed in a four-neck round bottom flask, and dried with hot nitrogen gas, and cooled for 30 minutes. plusInto the o-methyl chlorobenzene, a small amount of ether, iodine, heating to initiate the reaction. Diethyl ether was added dropwise to the reaction flask to make a total solvent amount of 1000 ml.The reaction temperature was controlled, reacted for 4 hours, cooled, and the resulting format reagent was used.(2) Preparation of intermediate 6-(2-chlorobenzoyl)tolueneFormat the reagent in diethyl ether solution (concentration 2.0M) 1000 ml, slowly drip into the ice bath containing 420.0 g(2.4 M) o-chlorobenzoyl chloride in xylene solution, the reflux reaction was continued for 36 hours, the reaction was completed, and the solvent was evaporated under reduced pressure. anti-The contents were poured into 1000 ml of ice-dilute hydrochloric acid, extracted twice with 1000 ml of toluene, and toluene was combined. Dry anhydrous magnesium sulfate, minusThe toluene was distilled off to give an intermediate 6-(2-chlorobenzoyl)toluene (425.6 g, yield: 92.5%).
References:
CN108530293,2018,A Location in patent:Paragraph 0041; 0042; 0044; 0045; 0046; 0047
615-37-2
288 suppliers
$5.00/5g
201230-82-2
1 suppliers
inquiry

3900-89-8
378 suppliers
$6.00/1g

4888-03-3
9 suppliers
inquiry
108-88-3
7 suppliers
$14.00/100ml
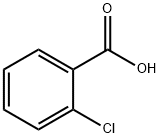
118-91-2
640 suppliers
$14.00/25g

4888-03-3
9 suppliers
inquiry
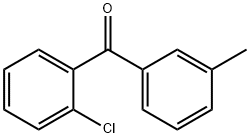
131822-46-3
12 suppliers
$294.00/1g
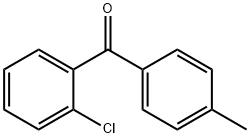
5953-00-4
16 suppliers
$45.00/5mg
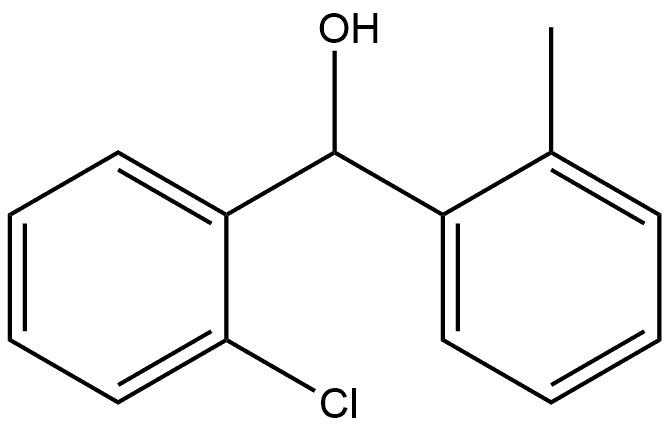
38493-62-8
2 suppliers
inquiry

4888-03-3
9 suppliers
inquiry