
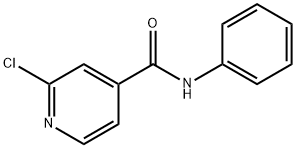
2-Chloro-3-methyl-N-methyl-N-phenylisonicotinamide synthesis
- Product Name:2-Chloro-3-methyl-N-methyl-N-phenylisonicotinamide
- CAS Number:133928-64-0
- Molecular formula:C14H13ClN2O
- Molecular Weight:260.72
80194-83-8
48 suppliers
$11.00/250mg

74-88-4
344 suppliers
$15.00/10g

133928-64-0
11 suppliers
inquiry
Yield:133928-64-0 93%
Reaction Conditions:
Stage #1: 2-chloro-N-phenylisonicotinamidewith n-butyllithium in tetrahydrofuran;hexanes at -78; for 1.23333 h;Inert atmosphere;
Stage #2: methyl iodide in tetrahydrofuran;hexanes at -78 - 20; for 21.1333 h;
Steps:
44.2
[01648] Step 2: Synthesis of 2-chloro-N,3-dimethyl-N-phenylpyridine-4-carboxamide[01649] To a stirred solution of 2-chloro-N-phenylpyridine-4-carboxamide (2.0 g, 8.6 mmol) in dry THF (40 ml) at -78°C under nitrogen, was added dropwise 1 .6M butyl lithium in n- hexanes ( 1 1 .8 ml, 18.9 mmol) over 24 minutes. The reaction mixture was stirred at -78°C for 50 minutes and then treated with iodomethane (1 .8 ml, 28 mmol) dropwise over 8 minutes. The reaction mixture was stirred at -78°C for 1 hour and then left to slowly reach room temperature and stirred for 20 hours. The reaction mixture was quenched with water (20 ml), and the layers were separated. The aqueous phase was extracted with CH2CI2 (3 x 20 ml). The combined organic phases were washed with brine (60 ml), dried (MgSC>4), filtered,concentrated in-vacuo and to give the title compound (2.2 g, 93%) as a thick brown oil. LC- MS 95%, m/z = 261 .0, 263.0; NMR (500 MHz, Chloroform-d) δ ppm 8.03 (d, J=4.89 Hz, 1 H) 7.23 (d, J=7.88 Hz, 2 H) 7.16 - 7.21 (m, 1 H) 7.02 (d, J=7.25 Hz, 2 H) 6.89 (d, J=4.89 Hz, 1 H) 3.51 (s, 3 H) 2.34 (s, 3 H).
References:
WO2012/142513,2012,A1 Location in patent:Page/Page column 427