
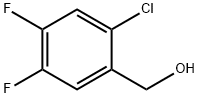
2-CHLORO-4,5-DIFLUOROBENZYL BROMIDE synthesis
- Product Name:2-CHLORO-4,5-DIFLUOROBENZYL BROMIDE
- CAS Number:874285-21-9
- Molecular formula:C7H4BrClF2
- Molecular Weight:241.46
288154-93-8
30 suppliers
$28.60/25MG

874285-21-9
18 suppliers
$66.70/1g
Yield:-
Reaction Conditions:
with hydrogen bromide in water at 8 - 50; for 1.16667 h;
Steps:
7
A solution of methyl 2-chloro-5-fluoro-4-methoxybenzoate (2.00 kg of99.04 wt % material, 9.06 moles, Example 6) in toluene (17.0 L) cooled to 13-15 °C wastreated with a 65 wgt % solution of Red-Al (sodium bis(2-methoxyethoxy) aluminumhydride, 2.95 L, 9.83 moles, 1.08 eq) over 1 hour while maintaining the temperature 13-17 °C. Sampling by HPLC at this point established that all of the starting material hadreacted.[00119] Remaining Red-Al was quenched by the addition of acetone (40 mL). Thereaction mass was transferred to a solution of 48% hydrobromic acid (19.0 L, 168 moles,18.5 eq) previously cooled to 8 °C. The addition took 40 min at <50 °C. The reactionmixture was heated to 50 °C and held 30 min at which point HPLC indicated 999:1 of 1-bromomethyl-2-chloro-5-fluoro-4-methoxybenzene to (2-chloro-5-fluoro-4-methoxyphenyl)methanol.[00120] The phases were separated and the organic phase washed successivelywith water five times (2.0 L) until the pH of the aqueous wash reached 5. The solution was filtered through a cartridge filter to produce 18.1 kg of solution, analyzed as 13.17 weight % l-bromomethyl-2-chloro-5-fluoro-4-methoxybenzene. This intermediate was mixed with acetic acid (195 mL, 3.4 moles, 0.36 eq). A solution of sodium cyanide (1780 g, 36.3 moles, 3.84 eq) and benzyltributyl ammonium chloride (195 g, 0.63 mole, 0.07 eq) in water (7.85 L) was added over 1 minute with vigorous stirring. This mixture was washed in with additional water (2.0 L) and the temperature was adjusted to 35 °C. Stirring was continued for 2.5 hours and HPLC sampled to indicate a (2-chloro-5-fluoro-4-methoxy-phenyl)-acetonitrile to 1 -bromomethyl-2-chloro-5-fluoro-4-methoxybenzene ratio of 1999:1. The phases were separated and the organic phase washed with water (18.5L). This batch was combined with another batch prepared on the same scale. The solution was concentrated by rotary evaporation at <50 °C until the level was ~12 L, at which point the concentration was continued but the level was maintained by the addition of isopropanol. This concentration procedure was continued until GC indicated the toluene content to be 2.09% v/v. A total of 18 L of isopropanol was required. The volume was diluted to 16.5 L with IP A and the solids dissolved by heating. The solution was cooled to 45 °C and the pressure reduced to 120 mmHg to distill out isopropanol while adding water .to maintain the volume. The temperature was maintained at 45-50 °C. A total of ~15 L water was charged over 5 hours before GC analysis indicated that the isopropanol level had been reduced to 5.7%. The slurry was cooled to ambient temperature overnight and the crystals collected by filtration. The cake was washed with water (5 L) over several washes and the crystals were vacuum dried at 50 °C (25" vacuum) over 4 days to produce 3.533 kg of 99.17 weight % purity (97 % corrected yield) of (2-chloro-5 -fluoro-4-methoxy-phenyl)-acetonitrile.
References:
WO2005/51954,2005,A2 Location in patent:Page/Page column 35; 36