
2-Chloro-4,6-diMethyl-3-nitropyridine synthesis
- Product Name:2-Chloro-4,6-diMethyl-3-nitropyridine
- CAS Number:89793-09-9
- Molecular formula:C7H7ClN2O2
- Molecular Weight:186.6
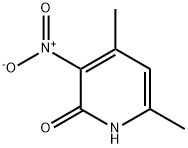
22934-13-0
25 suppliers
inquiry

89793-09-9
35 suppliers
inquiry
Yield:89793-09-9 90%
Reaction Conditions:
with trichlorophosphate at 95; for 3 h;
Steps:
1.2
A mixture of 4,6-dimethyl-3-nitro-2(1 H)-pyridinone (step 1 , 10.0 g, 29.7 mmol) in phosphorus oxychloride (35 mL, 187.3 mmol) was stirred at 95 °C for 3 h, then cooled to 45 °C. The excess amount of phosphorus oxychloride was removed by distillation under reduced pressure at 45 °C. The residue was cooled to room temperature, and diluted with dichloromethane (75 mL). The resulting solution was cooled to 0°C, and 2N hydrochloric acid (50 mL) was added dropwise into the solution. The organic layer was separated, and washed with 2N hydrochloric acid (4 x 25 mL), 2N aqueous NaOH (2 x 50 mL) and brine (50 mL). The organic phase was dried (MgSO4) and concentrated under reduced pressure to give 10.0 g (90%) of the title compound as white solids: 1H-NMR (CDCI3) δ 7.07 (1 H, s), 2.56 (3H1 s), 2.35 (3H, s).
References:
WO2006/95268,2006,A1 Location in patent:Page/Page column 28
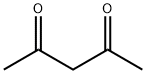
123-54-6
584 suppliers
$10.00/25ml

89793-09-9
35 suppliers
inquiry

22934-13-0
25 suppliers
inquiry

89793-09-9
35 suppliers
inquiry
