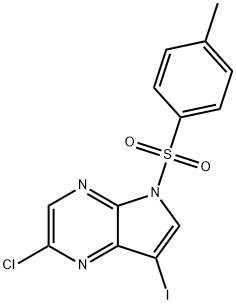
2-Chloro-7-iodo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine synthesis
- Product Name:2-Chloro-7-iodo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine
- CAS Number:889447-21-6
- Molecular formula:C13H9ClIN3O2S
- Molecular Weight:433.65
![2-Chloro-7-iodo-5H-pyrrolo[2,3-b]pyrazine](/CAS/GIF/889447-20-5.gif)
889447-20-5
50 suppliers
$290.00/100mg

98-59-9
593 suppliers
$9.00/5g
![2-Chloro-7-iodo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine](/CAS/20180703/GIF/889447-21-6.gif)
889447-21-6
2 suppliers
inquiry
Yield:889447-21-6 75%
Reaction Conditions:
Stage #1: 2-chloro-7-iodo-5H-pyrrolo[2,3-b]pyrazinewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2: p-toluenesulfonyl chloride in N,N-dimethyl-formamide at 20; for 18 h;
Stage #3: with water in N,N-dimethyl-formamide;
Steps:
I.1
Sodium hydride (140mg, 3.5mmol) was added to an ice-cold solution of 2-chloro-7- iodo-5H-pyrrolo[2,3-b]pyrazine (5) (820mg, 2.9mmol) in dimethylformamide (7ml) under nitrogen. After 30 minutes tosyl chloride (570mg, 3mmol) was added to the reaction mixture and the reaction mixture was stirred at room temperature for 18h. The reaction mixture was then quenched with water (~15ml). An off white solid was filtered off and dried in vacuo (950mg, 75%). MS(ES+) 434.
References:
WO2006/58074,2006,A1 Location in patent:Page/Page column 21