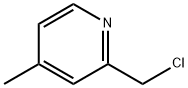
2-CHLOROMETHYL-4-METHYLPYRIDINE synthesis
- Product Name:2-CHLOROMETHYL-4-METHYLPYRIDINE
- CAS Number:38198-16-2
- Molecular formula:C7H8ClN
- Molecular Weight:141.6
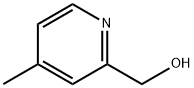
42508-74-7
77 suppliers
$85.00/50mg

38198-16-2
38 suppliers
inquiry
Yield:38198-16-2 72.1%
Reaction Conditions:
Stage #1: (4-methyl-2-pyridinyl)methanolwith thionyl chloride in dichloromethane; for 0.0833333 h;Heating / reflux;
Stage #2: with sodium hydrogencarbonate in diethyl ether;water;
Steps:
11.1.4
A mixture solution of (4-methyl-pyridine-2-yl)-methanol (410 mg, 3.33 mmol) described in Manufacturing Example 11-1-3, thionyl chloride (0.49 mL, 6.66 mmol) and methylene chloride (10 mL) was stirred under reflux for 5 minutes. The reaction solution was allowed to room temperature and concentrated under a reduced pressure. The resulting residue was partitioned into diethyl ether and saturated sodium bicarbonate solution. The organic layer was purified by silica gel column chromatography (ethyl acetate) to obtain the title compound (340 mg, 72.1%). 1H-NMR Spectrum (DMSO-d6) δ (ppm): 2.37 (3H, s), 4.72 (2H, s), 7.20 (1H, d, J=5.2 Hz), 7.38 (1H, s), 8.40 (1H, d, J=5.2 Hz).
References:
US2007/105904,2007,A1 Location in patent:Page/Page column 62

1122-45-8
31 suppliers
inquiry

38198-16-2
38 suppliers
inquiry
55485-91-1
10 suppliers
$369.00/1g

38198-16-2
38 suppliers
inquiry
108-47-4
215 suppliers
$9.00/1g

38198-16-2
38 suppliers
inquiry
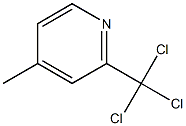
68265-89-4
0 suppliers
inquiry
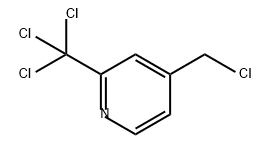
94126-96-2
0 suppliers
inquiry
