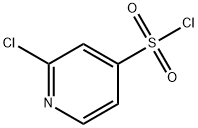
2-CHLOROPYRIDINE-4-SULFONYL CHLORIDE synthesis
- Product Name:2-CHLOROPYRIDINE-4-SULFONYL CHLORIDE
- CAS Number:1000933-25-4
- Molecular formula:C5H3Cl2NO2S
- Molecular Weight:212.05
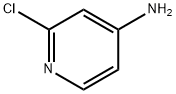
14432-12-3
541 suppliers
$5.00/5g

1000933-25-4
41 suppliers
inquiry
Yield: 49%
Reaction Conditions:
Stage #1:4-Amino-2-chloropyridine with hydrogenchloride;sulfur dioxide;acetic acid;trifluoroacetic acid;sodium nitrite in water at 0; for 1 h;
Stage #2: with sulfur dioxide;copper(l) chloride;copper dichloride at 0; for 1.5 h;
Steps:
59 2-Chloropyridine-4-sulfonyl chloride
4-Amino-2-chloropyridine (1.29 g, 10 mmol), TFA (10 mL), and con HCl (5 mL) was treated with NaNO2 (2.07 g, 30 mmol) in water (7.5 mL) at 0° C., then stirred for 1 h at 0° C.
The solution was filtered at -5° C. and added to a solution of CuCl (0.10 g, 0.7 mmol), CuCl2 (0.67 g, 3.9 mmol) in HOAc containing dissolved SO2 (60 mL) (prepared by bubbling SO2 gas through HOAc at rt for 2 h) at 0° C.
The reaction mixture was stirred at 0° C. for 1.5 h and diluted with DCM (50 mL).
The reaction was washed with ice-water (2*50 mL), sat'd NaHCO3 (2*50 mL), and brine (50 mL).
The organic layer was dried with Na2SO4, and concentrated to yield 2-chloropyridine-4-sulfonyl chloride (1.05 g, 49%); 1H NMR: (400 MHz, DMSO-d6) δ: 7.82-7.84 (m, 1H), 7.93 (s, 1H), 8.78-8.80 (m, 1H).
References:
FLATLEY DISCOVERY LAB;Cole, Bridget M.;Nugent, Richard A.;Smith, JR., Paul T. US2016/96835, 2016, A1 Location in patent:Paragraph 0108; 0109
73583-37-6
327 suppliers
$9.00/1g

1000933-25-4
41 suppliers
inquiry