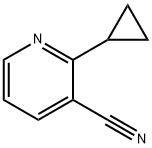
2-cyclopropylnicotinonitrile synthesis
- Product Name:2-cyclopropylnicotinonitrile
- CAS Number:921760-69-2
- Molecular formula:C9H8N2
- Molecular Weight:144.1732
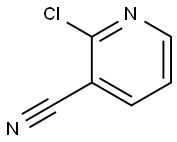
6602-54-6
490 suppliers
$6.00/10g
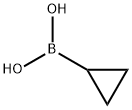
411235-57-9
427 suppliers
$6.00/1g

921760-69-2
9 suppliers
inquiry
Yield:921760-69-2 67.52%
Reaction Conditions:
with tripotassium phosphate tribasic;palladium diacetate;tricyclohexylphosphine in lithium hydroxide monohydrate;toluene at 100; for 5 h;Inert atmosphere;
Steps:
53.1 Step 1. Preparation of compound (53)-1
In the reaction flask, add (53)-0 (1.0 g, 7.2 mmol),Cyclopropylboronic acid (743 mg, 8.6 mmol), potassium phosphate (4.58 g, 21.6 mmol),Tricyclohexylphosphorus (202 mg, 0.72 mmol), palladium acetate (90 mg, 0.36 mmol), water (2 mL) and toluene (40 mL) were heated to 100°C under nitrogen and stirred for 5 hours. After the reaction was completed, anhydrous sodium sulfate was added to dry, the reaction solution was filtered through celite, the organic phase was concentrated, and the residue was applied to a silica gel column [eluent: petroleum ether-ethyl acetate (100:1-2:1) ], the eluent was collected, and the solvent was evaporated under reduced pressure to obtain a yellow solid (53)-1 (700 mg, 67.52%).
References:
WO2022/199599,2022,A1 Location in patent:Page/Page column 75