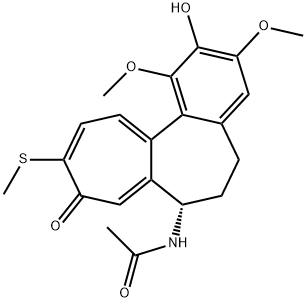
2-demethylthiocolchicine synthesis
- Product Name:2-demethylthiocolchicine
- CAS Number:87424-26-8
- Molecular formula:C21H23NO5S
- Molecular Weight:401.48

2730-71-4
74 suppliers
$33.00/5mg

87424-26-8
8 suppliers
inquiry
Yield:87424-26-8 36%
Reaction Conditions:
with sulfuric acid at 60; for 7 h;
Steps:
2-Demethylthiocolchicine (10)
Concentrated H2SO4 (98%,14 mL) was added to thiocolchicine 9 (2 g, 4.8 mmol). The mixture was stirred for 7 h at 60 C. The completion of the reaction was monitored by TLC using cyclohexane-AcOEt-95% EtOH(5 : 2 : 1) as the eluent. The resulting orange solution was poured onto ice (100 g), diluted with water (20 mL), brought to pH 5 using a 15% NaOH solution, and extracted with a CHCl3-PriOH mixture (3 : 1, 4×25 mL). The organic layer was dried over Na2SO4, and the solvent was distilled off under reduced pressure.The product was twice purified by silica gel column chromatography using a cyclohexane-AcOEt-95% EtOH mixture (5 : 2 : 1) as the eluent. The product was obtained in a yield of 0.7 g (36%) as yellow crystals, m.p. 178-185 C. 1H NMR (400 MHz,DMSO-d6), δ: 8.66 (s, 1 H, OH); 8.58 (d, 1 H, NH, J = 7.5 Hz);7.25 (d, 1 H, H(11), J = 10.5 Hz); 7.15 (d, 1 H, H(12), J = 10.5 Hz);7.02 (s, 1 H, H(8)); 6.68 (s, 1 H, H(4)); 4.29-4.40 (m, 1 H,H(7)); 3.82 (s, 3 H, OC(3)H3); 3.46 (s, 3 H, OC(1)H3); 2.52-2.57(m, 1 H, H(6)); 2.41 (s, 3 H, SCH3); 2.19 (td, 1 H, H(6),
References:
Fedorov, A. Yu.;Kuzmina, N. S.;Malysheva, Yu. B.;Mol’kova, E. A.;Otvagin, V. F.;Shchegravina, E. S.;Svirshchevskaya, E. V.;Zaburdaeva, E. A. [Russian Chemical Bulletin,2022,vol. 71,# 3,p. 564 - 571][Izv. Akad. Nauk, Ser. Khim.,2022,# 3,p. 564 - 571]
![Acetamide, N-[(7S)-5,6,7,9-tetrahydro-2,3-dihydroxy-1-methoxy-10-(methylthio)-9-oxobenzo[a]heptalen-7-yl]-](/CAS/20210305/GIF/51296-12-9.gif)
51296-12-9
0 suppliers
inquiry
18107-18-1
208 suppliers
$33.00/5g

87424-26-8
8 suppliers
inquiry

87424-25-7
29 suppliers
$76.00/2mg

64-86-8
642 suppliers
$13.00/100mg

87424-26-8
8 suppliers
inquiry